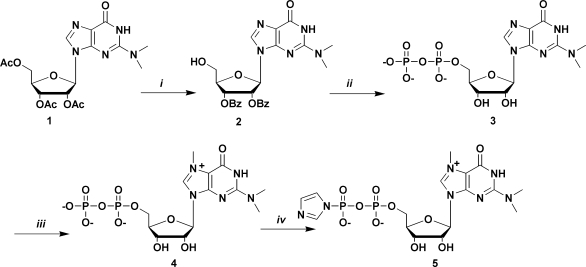
Scheme 1.
Synthesis of the m3G 5′-pyrophosphateimidazolide. Reagents and conditions: (i) a: Py/NH3aq sat., 3 h, r.t.,; b: MMTrCl, Py/DMF, 24 h, r.t.; c: BzCl, Py, r.t., 24 h, D 80% AcOH, r.t., 5 h; (ii) a: salicyl chlorophosphite, r.t., 15 min; b: tri-n-buthylammine pyrophosphate, DMF, r.t, 20 min; c: I2, Py/H2O, r.t, 15 min; d: ethylenediamine, r.t., E NH3aq sat., 0°C 48 h; (iii) MeI, DMF, 40°C, 5 h; (iv) imidazole, triphenylphosphine, di-2-pyridyldisulphide, DMF 24 h. Py, pyridine; MMTrCl, monometoxy trityl chloride; DMF, dimethylformamide; r.t. = room temperature; BzCl, benzoyl chloride; AcOH, acetic acid; MeI, methyl iodide.