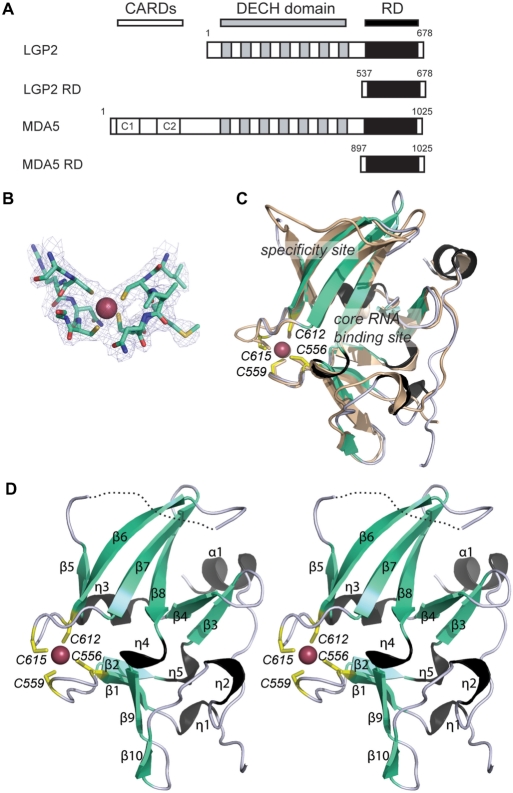
Figure 1.
Structure of the RD of human LGP2. (A) Scheme of the domain arrangement in LGP2 and MDA5. The two N-terminal CARD motives of MDA5 are depicted in white, the DExH box helicase domains are shown in gray and the RDs in black. Numbering of the RDs is according to the constructs used for structural and biochemical studies. (B) Portion of the final 2Fo−Fc electron density (1 s contour) at the mercury-binding site superimposed with the final model. (C) Superimposition of ribbon models of LGP2 RD (cyan, black and yellow) and RIG-I RD (wheat) with highlighted secondary structure and metal-binding site. Possible RNA binding and specificity sites are assigned. (D) Stereo image of a ribbon model of LGP2 RD with highlighted and annotated secondary structure, mercury ion (magenta sphere) and annotated mercury-coordinating cysteines (yellow sticks).