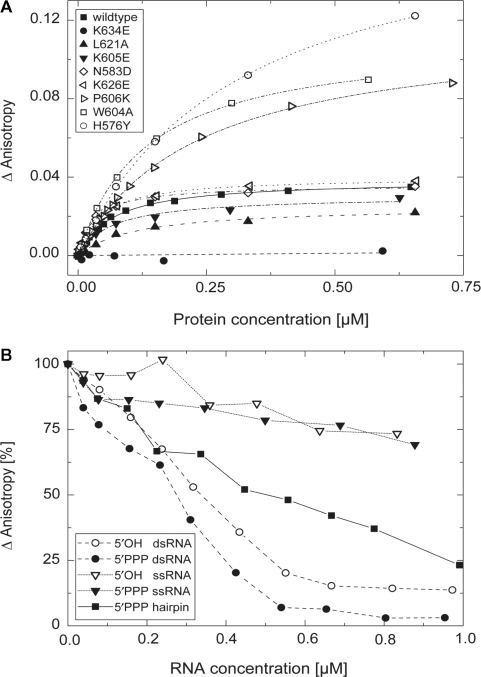
Figure 3.
Binding of dsRNA to LGP2 RD. (A) Fluorescence anisotropy changes of a 5′-Alexa Fluor 488-labeled 25 bp RNA duplex (37 nM) in response to titration with wild-type LGP2 RD (filled square, Kd=68 ± 6 nM) and various mutants, respectively. Control mutation N583→D (open diamond, Kd=38 ± 4 nM) located on the convex site of RD and mutation K626→E (open left-facing triangle, Kd=51 ± 5 nM) do not show significantly altered dsRNA binding affinity. Affinities for mutants L621→A (filled up-facing triangle, Kd=165 ± 26 nM) and K605→E (filled down-facing triangle, Kd=140 ± 16 nM) are slightly decreased, while a mutation of K634→E (filled circle) completely suppresses binding. A decrease of binding affinity, but increase in maximum reached anisotropy signal is seen for P606→K (open right-facing triangle, Kd=230 ± 10 nM), W604→A (open square, Kd=136 ± 6 nM) and H576→Y (open circle, Kd=304 ± 10 nM). (B) Competition of binding of an Alexa Fluor 488-5-U-labeled hairpin RNA (in vitro transcriped, 40 nM) to LGP2 RD (470 nM) by stepwise addition of different non-fluorescent RNA species (synthetic 5′OH/5′OH dsRNA: 19 bp; 5′PPP/5′OH dsRNA: 19 bp; 5′OH ssRNA: 19n; 5′PPP ssRNA: 19n; and in vitro transcribed 5′PPP hairpin: 18 bp ± 4n) followed by fluorescence anisotropy. Data points were connected for better outline.