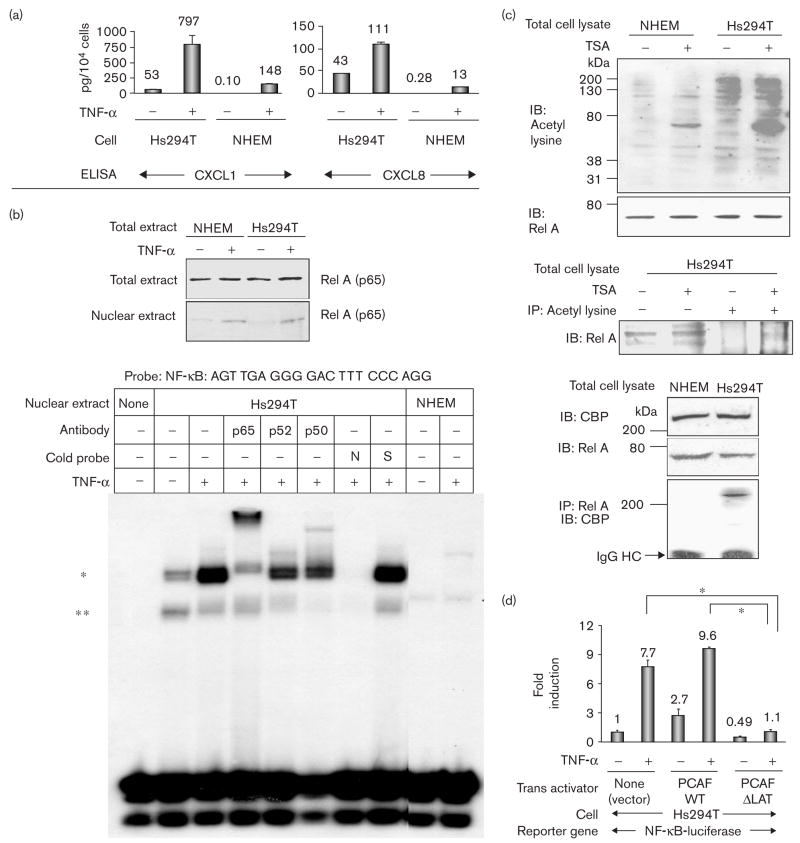
Fig. 1.
Rel A-DNA binding and acetylases are highly activated in Hs294T metastatic melanoma cells compared with normal human epidermal melanocytes (NHEM). (a) Hs294T cells and NHEM were incubated in normal growth media, washed twice with serum-free medium (SFM), and then incubated with/without tumor necrosis factor-α (TNF-α) (10 ng/ml) in SFM for 4 h. Media were collected, centrifuged to eliminate cell debris, and aliquots were subjected to enzyme-linked immunosorbent assay (ELISA) to determine the presence of CXCL1 and CXCL8. The ELISA was repeated thrice and the values were normalized in the unit of pg/104 cells. (b) Top: Hs294T cells and NHEM were incubated in normal growth media, washed twice with SFM, incubated with/without TNF-α (10 ng/ml) in SFM for 1 h, and lysed to obtain total and cytoplasm/nuclear fractionated proteins. Proteins (40 μg) were subjected to immunoblot analysis with an αRel A antibody. Bottom: nuclear proteins (0.5 μg) were subjected to electrophoretic mobility shift assay (EMSA) with the probe of Nuclear factor kappa beta (NF-κB) (promega) as indicated. (*) EMSA band that significantly increased with TNF-α and was shifted by coincubation with the αRel A antibody; (**) EMSA band that was mainly shifted by coincubation with the αP50 antibody; N: 50-fold excess of unlabeled NF-κB consensus oligonucleotide; S: 50-fold excess of unlabeled Sp-1 consensus oligonucleotide. (c) Top: the total cell lysates from Hs294T and NHEM with/without 500 nmol/l trichostatin A (TSA) were subjected to immunoblot analysis with α-acetyl-lysine and αRel A antibodies. IB: immunoblot analysis. Middle: the total cell lysates from Hs294T with/without 500 nmol/l TSA were subjected to immunoblot analysis (lanes 1, 2). The total cell lysates from Hs294T with/without 500 nmol/l TSA were also subjected to immunoprecipitation with α acetyl-lysine antibody followed by immunoblot analysis with αRel A antibodiy (lane 3, 4). Bottom: the total cell lysate from Hs294T and NHEM were subjected to immunoblot analysis with αCBP and αRel A antibodies. Cell lysates were also immunoprecipitated with αRel A antibody, and the immunoprecipitated complexes were subjected to immunoblot analysis with αCBP antibody. IP: immunoprecipitation; IgG HC: immunoglobulin G heavy chain. (d) Cells (4 × 105 cells) were seeded in 24-well plates and transfected with 0.2 μg/well of NF-κB-luciferase reporter plasmid and 0.02 μg/well of pNull-Renilla luciferase reporter plasmid in a complete growth medium. Cells were also co-transfected with 0.2 μg/well of one of the following trans-activators: (i) PCAF-WT, (ii) PCAF-ΔLAT or (iii) pcDNA3 empty control vector. Cells were incubated in SFM for 16 h and then further incubated with/without TNF-α (10 ng/ml) for 4 h. NF-κB-luciferase reporter activity was divided by Renilla luciferase activity to normalize the transfection efficiency. Standard deviations of the mean fold induction were calculated from the relative luciferase activity from the three wells. The assays were done thrice. *P < 0.002 (Student’s t-test).