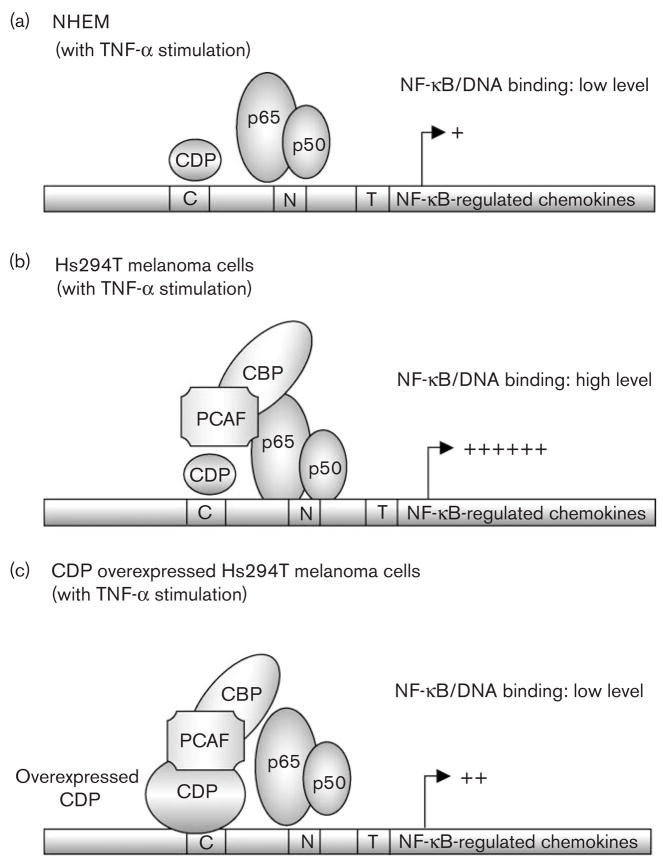
Fig. 5.
Hypothetical model: CCAAT displacement protein (CDP) interacts with lysine acetyl transferase, CBP/PCAF and interferes with the activation of nuclear factor-kappa beta (NF-κB). (a) Model of NF-κB-regulated transcription in normal human epidermal melanocytes (NHEM): NF-κB proteins (Rel A/p50 dimers) translocate into the nucleus after tumor necrosis factor α (TNF-α) stimulation. The binding of NF-κB proteins to DNA, however, is reduced in NHEM as compared with Hs294T cells. This may be due to the lack of interaction between NF-κB and the protein acetylase CBP/PCAF complex. (b) Model of NF-κB-regulated transcription in Hs294T melanoma cells: NF-κB proteins (Rel A/p50 dimers) translocate into the nucleus after TNF-α stimulation. NF-κB proteins bind to its consensus sequences in the nucleus, and this binding is possibly stabilized by the protein acetylase complex CBP/PCAF. (c) Model of NF-κB-regulated transcription in Hs294T melanoma cells with over-expressed CDP: NF-κB proteins (Rel A/p50 dimers) translocate into the nucleus after TNF-α stimulation. Over-expressed CDP stably binds to DNA and interacts with CBP/PCAF, which may lead to the sequestration of CBP/PCAF from Rel A and to the unstable DNA binding of Rel A/p50 complex.