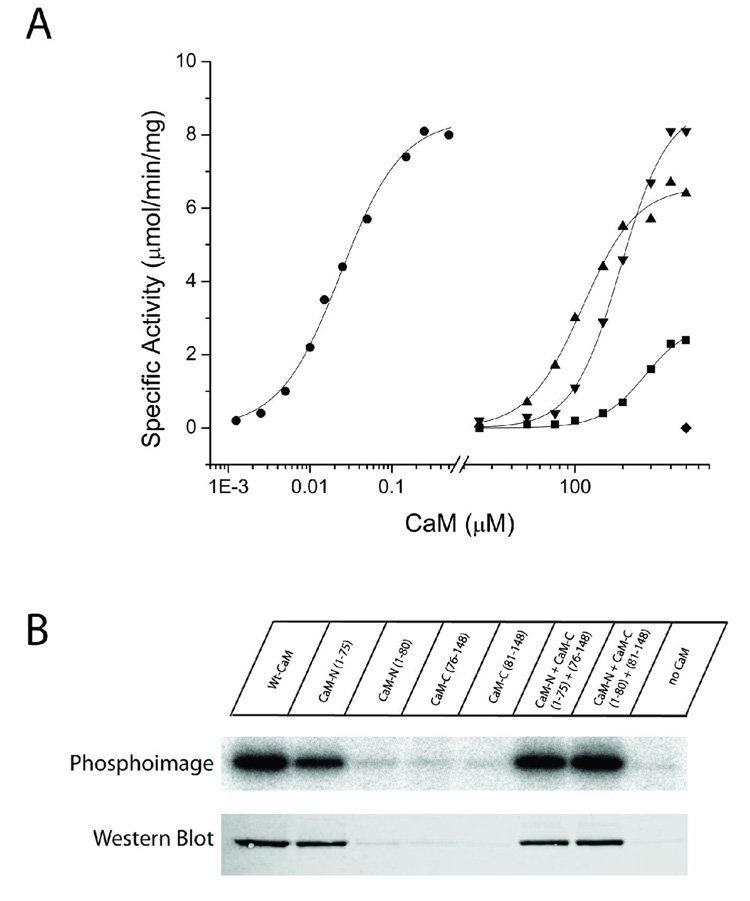
Figure 4.
Substrate phosphorylation and autophosphorylation induced by half-CaMs. A) Each CaM variant was assessed for its ability to activate CaMKII in reactions containing 25 mM HEPES, pH 7.4, 50 mM KCl, 0.4 mM DTT, 10 mM MgCl2, 4 mM CaCl2, 100 µM ATP, 50 µM syntide, and 2 µCi of 32P-ATP. Reactions were initiated by the addition of 10 ng of CaMKII, incubated for 1 min at 30°C, and terminated by spotting an aliquot on P81 paper and plunging the paper into phosphoric acid. Each reaction was performed in duplicate and separate experiments were repeated a minimum of 2 times. The CaM constructs shown are: wtCaM (circles), CaMN1–75 (squares), CaMN1–75 + CaM 76–148 (triangles), CaMN1–80 + CaMC81–148 (upside down triangles). CaMN1–80, CaMC76–148 and CaMC81–148 were inactive up to the indicated concentration (diamond). B) Each CaM variant was assessed for its ability to induce autophosphorylation of CaMKII. The reaction mixture contained 25 mM HEPES, pH 7.4, 50 mM KCl, 0.4 mM DTT, 10 mM MgCl2, 4 mM CaCl2, 100 µM ATP, and 2 µCi of 32P-ATP. Reactions were initiated by the addition of 100 ng of CaMKII and were allowed to react for 10 min on ice. The reactions were terminated by the addition of SDS-sample buffer and 40 ng of CaMKII from each reaction were loaded and run on a 10% SDS-PAGE gel. Following electrophoresis, staining and destaining, 32P incorporation was assessed by exposing the gel to a phosphor screen for 5 hr and then analyzing the screen on a Typhoon imaging system. Identical samples were also analyzed by Western blot with a rabbit polyclonal antibody specific for phospho-Thr286. The primary antibody was detected with an Alexa568-labeled anti-rabbit antibody and the signal detected using the fluorescence mode of the Typhoon imaging system.