Fig. 1.
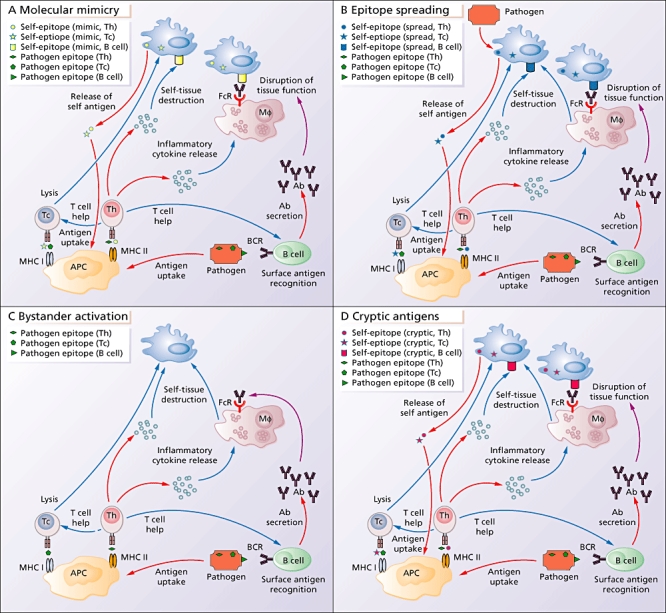
Mechanisms by which pathogens may cause autoimmunity. (a) Molecular mimicry occurs when pathogen-derived epitopes are cross-reactive with self-derived epitopes. Pathogen-derived epitopes are taken up by antigen-presenting cells (APCs) and presented to cytolytic T cells (Tc) via major histocompatibility complex (MHC) class I or to helper T cells (Th) via MHC class II. T cells activated by pathogenic epitopes that are cross-reactive with self-epitopes can then damage self-tissue via lysis (Tc) or release of cytokines (Th). Cytokines released by activated Th cells can activate macrophages (Mφ) or provide help to B cells. Pathogen-derived surface antigens are recognized by a B cell's B cell receptor (BCR), which triggers the secretion of antibodies. These antibodies can cause damage by binding to cross-reactive epitopes on the surface of tissues and disrupting tissue function, or the Fc portion of the antibody can bind simultaneously to the Fc receptor (FcR) on Mφ; this will trigger the Mφ to produce tissue-damaging cytokines. Damaged tissue will release more cross-reactive antigens, which will be taken up by APCs, propagating further damage. (b) In epitope spreading, the immune response to a persisting pathogen, or direct lysis of self-tissue by the persisting pathogen, causes damage to self-tissue. Antigens released from damaged tissue are taken up by APCs, and this initiates an immune response directed towards self-antigens. (c) In bystander activation, the various parts of the immune system respond to the invading pathogens. The inflammatory environment triggered by this response damages self-tissue in an antigen non-specific manner, and in addition triggers non-specific activation of immune cells. (d) In contrast to dominant antigenic determinants, subdominant cryptic antigens are normally invisible to the immune system. The inflammatory environment that arises after infection can induce increased protease production and differential processing of released self-epitopes by APCs.
