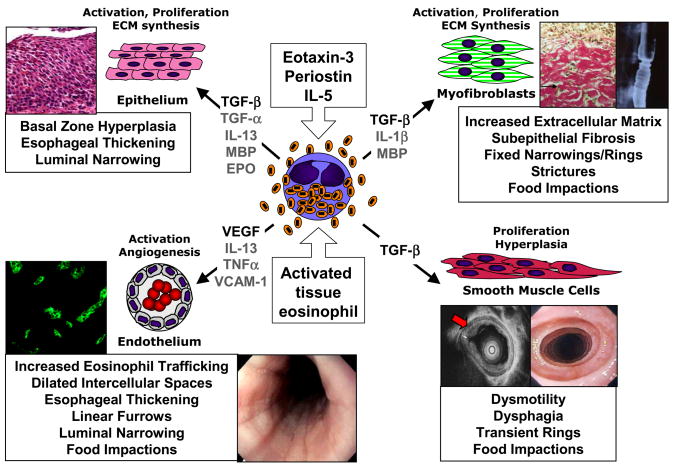
Figure 1. Eosinophil induction of esophageal remodeling and fibrosis in EE: relationships to endoscopic and histologic pathologies.
Eosinophil activation during recruitment to the esophagus occurs in response to eotaxin-3, periostin, IL-5 and interactions with vascular endothelium, epithelium and fibroblasts, leading to their expression of fibrogenic factors such as TGF-β. Eosinophil-expressed TGF-β and granule proteins (MBP, EPO) induce epithelial basal zone hyperplasia, contributing to esophageal thickening and luminal narrowing. Eosinophil-derived TGF-β induces fibroblast activation, with transdifferentiation to myofibroblasts and consequent over-production of ECM leading to subepithelial fibrosis, fixed narrowings/rings, strictures and food impactions. Alternatively, TGF-β expressed by eosinophils or MBP/EPO damaged epithelium itself may induce epithelial to mesenchymal (myofibroblast) transition (EMT) contributing to subepithelial fibrosis. Eosinophil-expressed TGF-β may induce smooth muscle cell hypertrophy/hyperplasia leading to thickening of the esophageal muscularis propria, contributing to dysmotility, dysphagia, transient rings and non-stricture food impactions. Eosinophil expression of VEGF likely supports increased angiogenic responses of vascular endothelium with VCAM-1 activation by IL-13 and TNF-α, contributing to increased eosinophil trafficking, dilated intercellular spaces, esophageal thickening, furrowing, luminal narrowing and non-stricture food impactions.