Figure 7.
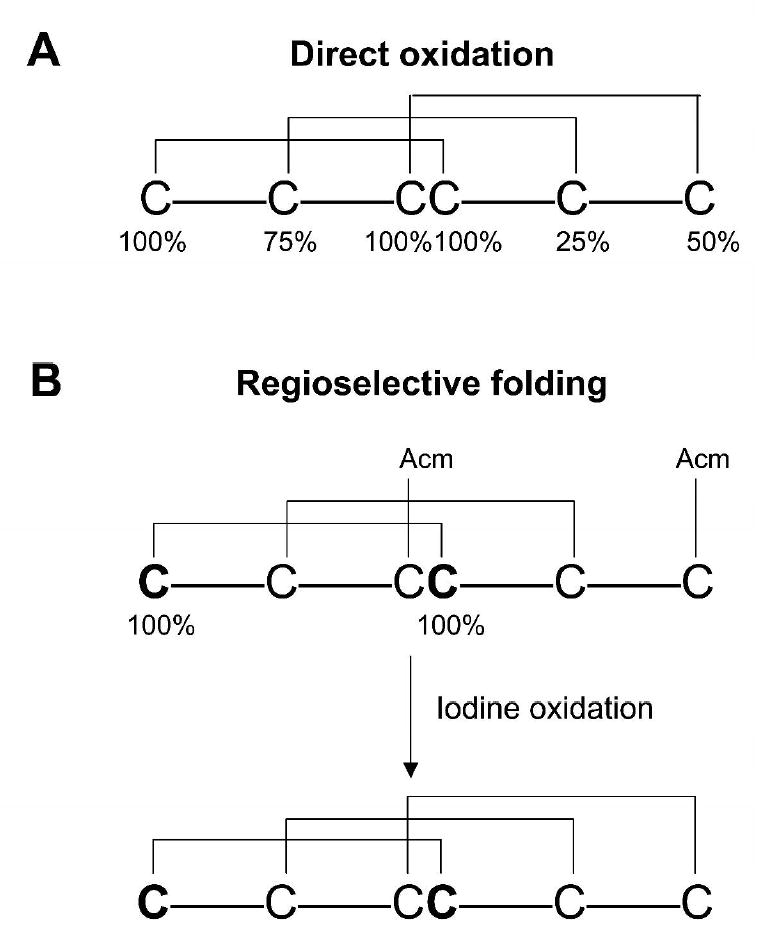
General strategies for NMR-based mapping of disulfide bridges in inhibitory cystine knot (ICK) peptides. (A) one-step folding strategy with labeling levels as indicated: CI-100%, CII-75%, CIII/CIV- 100%, CV-25%, and CVI-50% 15N/13C. The 100% sites are differentiated using triple resonance NMR connectivities while the remaining isolated sites are differentiated by enrichment levels. An alternative strategy would be to introduce 15N- and/or 15N,13C- labeled amino acids adjacent to isolated cysteines expanding the NMR capability. (B) two-step folding strategy with labeled cysteines: 100% labeled with 15N/13C and orthogonal cysteine protection. Since the first folding step involves the direct oxidation of four cysteines, three products are possible, only one having two native disulfide bridges. The 15N/13C labeled pair of cysteine residues that form one of the native disulfide bridges allows a rapid selection of the correctly folded isomer to be further oxidized.
