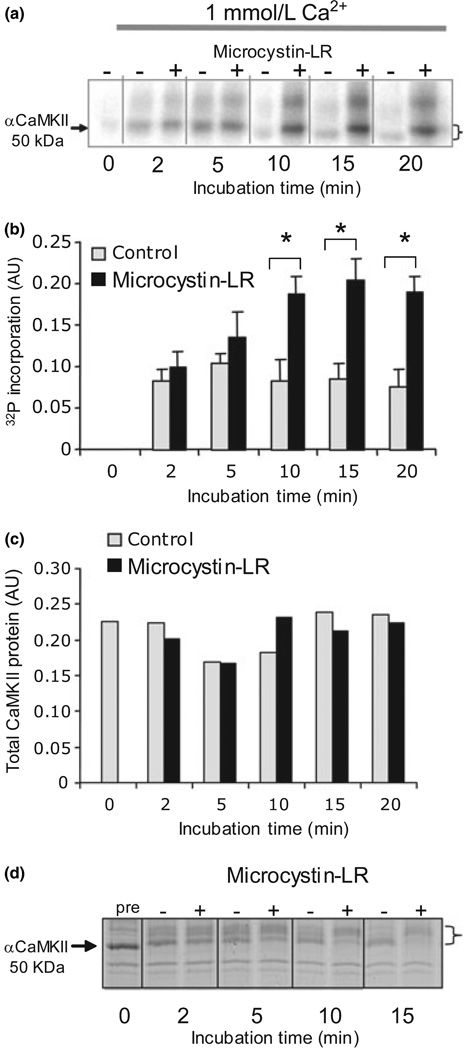
Fig. 1.
Phosphorylation of post-synaptic density (PSD) -bound Ca2+/calmodulin-dependent protein kinase II (CaMKII) is controlled by the balance of kinase and phosphatase activities. (a) 32P incorporation into PSD CaMKII under standard conditions in 1 mmol/L Ca2+ increases in Ca2+/CaM but plateaus at a lower level in reactions without microcystin-LR. Phosphorylation is accompanied by a shift in apparent molecular weight (indicated by a bracket). The higher molecular weight phospho-band that undergoes a similar shift and dephosphorylation is βCaMKII. (b) Phospho-CaMKII band intensity on the autoradiogram was quantified from three separate experiments using a phosphor-imager. After 10 min, there was a significant (p < 0.05, anova) difference between (+) and (−) microcystin-LR values. (c) Total kinase, quantified from parallel blots probed with the anti-αCaMKII (1 : 1000), did not change significantly. (d) Coomassie stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis profile of PSD proteins phosphorylated under standard conditions with 1 mmol/L Ca2+ showing that Ca2+/CaM initially shifts the apparent molecular weight of aCaMKII (indicated by arrow) to a higher value, followed by a return towards the unshifted value. The presence of microcystin-LR (+) blocked downward shift compared with sample in which there was no microcystin-LR (−). The magnitude of the shift is indicated by a bracket.