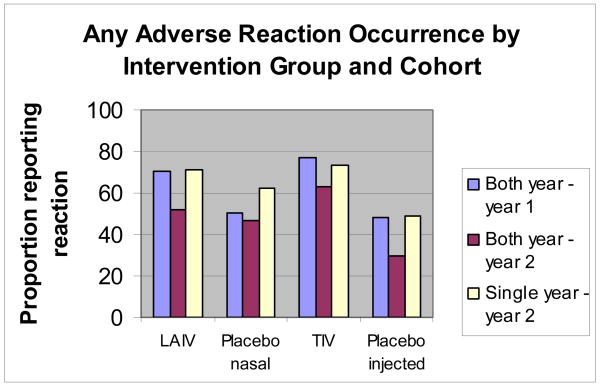
Figure 1. The proportions of participants reporting occurrence of any (one or more) adverse reactions by vaccine or placebo group, and cohort (both year versus single year participants).
LAIV (live-attenuated influenza vaccine); placebo nasal (saline in year 1 and normal allantoic fluid in year 2); TIV (inactivated vaccine); placebo injected (saline both years)