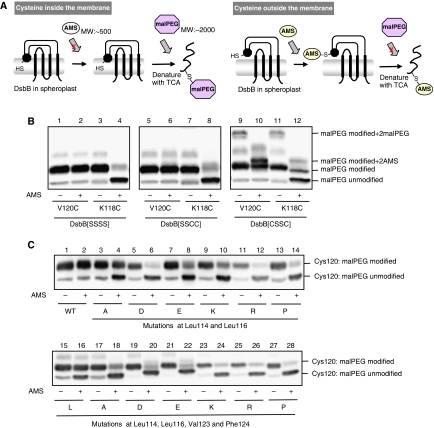
Figure 7.
Membrane association of the horizontal helix in wild-type and mutant DsbB. (A) Schematic depiction of AMS alkylation and malPEG counter-alkylation assay. Membrane-buried cysteine is not modified with AMS, a membrane-impermeable alkylating agent, in the first alkylation step. After denaturation with TCA and solubilization in SDS, however, it is exposed to the aqueous phase and modifiable with malPEG. In contrast, cysteine outside the membrane is subject to the first-step AMS alkylation and, hence, not to the second-step malPEG alkylation. The AMS (∼0.5 kDa) and the malPEG (∼2 kDa) modifications can be discriminated by the different extents of mobility retardation in SDS–PAGE. A black circle in spheroplast DsbB indicates the horizontal helix. (B) Different locations of Val120 and Lys118 relative to the membrane. [SSSS], [SSCC] and [CSSC] forms of spheroplast DsbB–myc proteins, in which either Val120Cys or Lys118Cys mutation was introduced, were subjected to the AMS alkylation and malPEG counter-alkylation assay shown in (A). MalPEG-modified species were separated from malPEG-unmodified species by SDS–PAGE (12.5%) and detected by western blot analysis with an anti-myc antibody. Extra bands observed for DsbB[CSSC] represent species having malPEG- or AMS-modified Cys41 and Cys130 as well as malPEG-modified Cys120 (or Cys118). (C) AMS alkylation and malPEG counter-alkylation assays for a series of DsbB[SSSS] derivatives having a single cysteine at position 120. Additionally, they had the indicated mutations. Detection was carried out as addressed in (B).