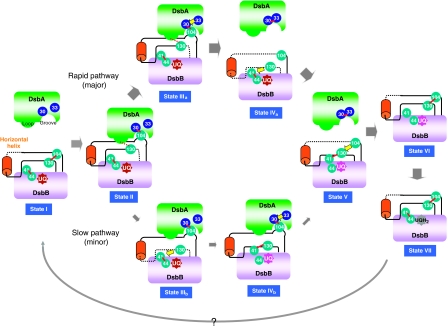
Figure 8.
Conformational transitions of DsbB for efficient DsbA oxidation. DsbB-mediated DsbA oxidation reaction is divided into seven states (I–VII). In each state, a disulphide bond and a flexible segment of DsbB are shown with a red line and a black dotted line, respectively. The present crystal structure of DsbB(Cys41Ser) represents the structure of the initial state (state I) in that it contains the Cys104–Cys130 disulphide. The DsbB–DsbA complex, the structure of which has now been updated, corresponds to state II, in which the Cys104-containing DsbB segment is pulled into the deep hydrophobic groove of DsbA to separate Cys104 from Cys130. Additionally, the Cys130-neighbouring peptide interacts with the Phe63–Gly65 loop of DsbA, as shown by a red dotted line. It is inferred that the membrane-associated horizontal helix of DsbB has a key function in properly controlling the motion of the Cys130-containing loop. In the next stage, a productive race between Cys33(DsbA) attack against the intermolecular disulphide (leading to the rapid pathway) and the Cys130(DsbB) attack against the Cys41–Cys44 disulphide (leading to the slow pathway) takes place. Cys130 approach to Cys41–Cys44 disulphide is inhibited in state II, presumably due to local contact between the Cys130-neighbouring segment and the Phe63–Gly65 loop of DsbA (red dotted line), explaining the major rapid and minor slow pathways (see Discussion for more details). In either pathway, state V with the Cys41–Cys130 interloop disulphide and the extremely flexible Cys104-containing loop is generated, as revealed by the NMR analysis of DsbB[CSSC] (Zhou et al, 2008). Cys104 then attacks Cys41–Cys130 disulphide, resulting in Cys104–Cys130 disulphide and reduced Cys41 and Cys44 (state VI). Two electrons thus moved onto the Cys41–Cys44 pair are accepted by UQ through the formation of the Cys44–UQ CT complex and covalent adduct, leading to the regeneration of fully oxidized DsbB (state VII). The reaction turns over through an unknown mechanism of UQ exchange.