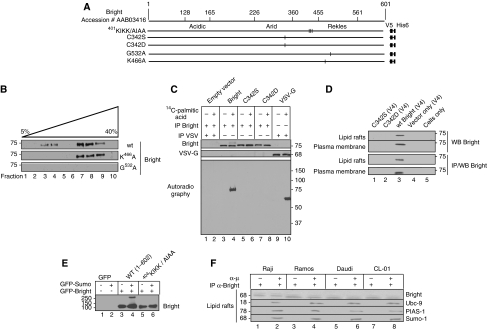
Figure 3.
Entry and exit of Bright from lipid rafts requires nucleocytoplasmic shuttling, alternative post-translational modifications, but not association with Btk. (A) Schematic of Bright indicating domains and positions of substitution mutations. (B) Cytoplasmic-nuclear shuttling is a requirement for Bright's inclusion into lipid rafts. NIH/3T3 fibroblasts were transfected with constructs encoding wild type and nuclear export signal-defective (NES, G532A) and nuclear localisation signal-defective (NLS, K466A) Bright. Fractions from discontinuous (5–40%) sucrose gradient purification of lipid rafts were analysed by western for Bright. (C) Palmitoylation of Bright requires cysteine 342. Bright constructs (indicated at the top) were transfected into Cos-7 cells, and after 48 h, were incubated as indicated with 14C palmitic acid. Whole cell lysates were subjected to immunoprecipitation using antibodies against Bright and VSV as indicated (left). SDS–PAGE separated proteins were transferred onto nitrocellulose, and the metabolic incorporation of 14C palmitic acid was determined by autoradiography. Solvent control is indicated by −. (D) Specification of Bright to lipid rafts requires cysteine 342. NIH/3T3 fibroblasts were transfected with constructs indicated in the figure. Crude plasma membrane and lipid rafts, prepared as described in previous legends, were analysed by anti-Bright western blotting. (E) Sumo-I modification of Bright is lost after mutation of 401KIKK. Cos-7 cells were transfected with GFP-SUMO-I and GFP-Bright expression constructs, as indicated. Whole cell lysates were prepared using RIPA buffer and analysed by western using α-Bright anti-serum. An arrow points to a GFP-Sumo-I conjugated species of GFP-Bright. (F) Bright forms a transient, stimulation-specific complex with Sumo-I-conjugation enzymes PIAS1 and Ubc9 in lipid rafts. Indicated B cells (∼108) were stimulated mildly (30 s; 100 ng α-μ). Lipid rafts were collected on gradients, subjected to immunoprecipitation with anti-Bright antiserum, and then analysed by western using the antibodies indicated.