Abstract
Despite several decades of research, the epigenesis of behavioural and brain lateralization is still elusive, although its knowledge is important in understanding developmental plasticity, function and evolution of lateralization, and its relationship with developmental disorders. Over the last decades, it has become clear that behavioural lateralization is not restricted to humans, but a fundamental principle in the organization of behaviour in vertebrates. This has opened the possibility of extending descriptive studies on human lateralization with descriptive and experimental studies on other vertebrate species. In this review, we therefore explore the evidence for the role of genes and environment on behavioural lateralization in humans and other animals. First, we discuss the predominant genetic models for human handedness, and conclude that their explanatory power alone is not sufficient, leaving, together with ambiguous results from adoption studies and selection experiments in animals, ample opportunity for a role of environmental factors. Next, we discuss the potential influence of such factors, including perinatal asymmetrical perception induced by asymmetrical head position or parental care, and social modulation, both in humans and other vertebrates, presenting some evidence from our own work on the domestic chick. We conclude that both perinatal asymmetrical perception and later social modulation are likely candidates in influencing the degree or strength of lateralization in both humans and other vertebrates. However, in most cases unequivocal evidence for this is lacking and we will point out further avenues for research.
Keywords: cerebral asymmetry, behavioural lateralization, development, handedness, plasticity, genetic models
1. Introduction
Lateralization of brain and behaviour refers to the fact that the hemispheres of the brain differentially control behaviour. It is also known as hemispheric or cerebral asymmetry/specialization (Vallortigara & Rogers 2005). At the behavioural level, it is often expressed in side biases for motor output, perception and information processing. For a long time, lateralization was considered unique to humans, but recently it has become clear that lateralization is a fundamental characteristic of the organization of brain and behaviour in vertebrates (Vallortigara & Rogers 2005). Animal models open new and exciting perspectives for understanding the function and evolution and provide the opportunity to experimentally study the causes and consequences of lateralization.
It is highly likely that such a fundamental aspect of brain and behaviour is under the control of genetic encoding. However, this does not exclude an important role for environmental factors in the development and expression of lateralization. The debate whether behavioural and brain lateralization is caused by genetic or environmental factors has been long-standing (Annett 1978b; Laland et al. 1995; Provins 1997; Bishop 2001). Insight into the epigenesis of lateralization is highly relevant to understand both its evolution and possible constraints on plasticity as well as its adaptive flexibility and pathologies. By describing correlations between genetic information, environmental factors and the development or expression of lateralization, or by manipulating genetic and environmental factors using animal models, such insights can be acquired.
Especially in the psychological literature, there is some consensus about the genetic heritability of lateralization. This is mainly based on the distribution and genetic modelling of handedness in humans. Handedness is heritable as it runs in families. Only 7.6 per cent of the children of two right-handed parents are left-handed. This percentage increases to 19.5 per cent if one of the parents is left-handed and to 54.5 per cent if both the parents are left-handed (Rife 1940). Heritability estimates vary between 0.23 and 0.66 (Denny & O' Sullivan 2007). However, these data are no hard evidence for a genetic basis for the degree or direction of lateralization in itself. Traits may run in families owing to exposure to environmental factors that are more similar within than between families and other forms of non-genomic inheritance. Furthermore, heritability estimates can be influenced by these factors too, and can differ greatly depending on the environment in which the data were obtained.
In this paper, we review the evidence for genetic and environmental influences on brain and especially behavioural lateralization in humans and other animal species. We focus on handedness since this might be more sensitive to (especially post-natal) environmental factors than lateralization of cognitive functions. We will first discuss the explanatory power of the existing genetic models for human handedness, including their strengths and weaknesses followed by what is known of genetic influences on lateralization in other animal species. Next, we will focus on environmental influences and review evidence for humans and other vertebrate species. Section 4 summarizes and synthesizes both sections and offers suggestions for future research.
2. Explanatory power of genetic models
One of the most common ways to investigate lateralization in humans is measuring handedness in combination with cerebral dominance for speech for which several genetic theories have been proposed. We will briefly describe the features of the main genetic models and the findings that challenge the hypothesis that handedness is determined genetically. For each of these potential problems, we will explore to what extent environmental factors may be an alternative to the genetic explanation.
(a) Models of genetic transmission of handedness
Although offspring of left-handed parents are more likely to be left-handed than offspring of right-handed parents, right-handed offspring can be produced by two left-handed parents (Rife 1940; McManus & Bryden 1992). The classical Mendelian approach incorporating a recessive allele for left-handedness (Jordan 1911) was therefore discarded.
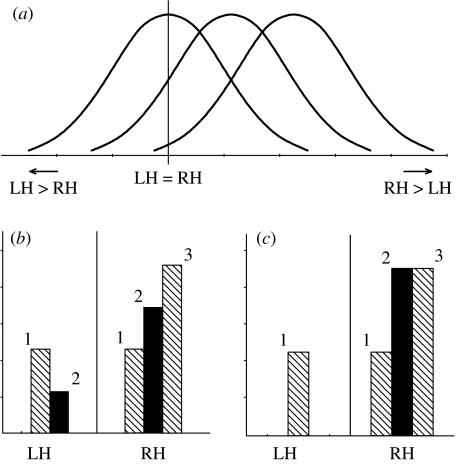
Subsequently, several other single-locus models were proposed. These models do not propose the existence of genes encoding for right- or left-handedness, but alleles for right-handedness (in combination with left-hemispheric dominance for language), and handedness (and language) becoming left- or right-lateralized by chance (Annett 1972, 1985, 2002; McManus 1985a, 1999; Klar 1996). This is to account for the finding that left-handers can be lateralized for language in either direction. In Annett's theory, a normal distribution (arising from environmental influences) of the difference in skill between the two hands exists. A ‘right’ allele, which encodes for left-cerebral dominance for speech, shifts this distribution to the right (increasing right- over left-hand skills; figure 1a). An individual with low left-hand skills and high right-hand skills is therefore likely to become right-handed (but not necessarily so if environmental factors, such as social pressures are high). By contrast, the right allele in McManus' and Klar's models encodes directly for right-hand preference and left-cerebral dominance (figure 1b,c, respectively). In these two models, homozygous individuals for the ‘chance’ allele (no right allele present) will be left- or right-handed with language left- or right-lateralized all with equal probabilities, whereas in Annett's model the skill distribution is centred around zero with approximately 50 per cent of these individuals better skilled with the right hand and 50 per cent with the left hand. Depending on the theory, heterozygotes become either right-handed (Klar 1996) or have an increased chance of becoming right-handed (Annett 1972, 1975; McManus 1985a, 1999). Homozygotes for the right allele will be right-handed according to McManus (1985a, 1999) and Klar (1996); in Annett's model (1972, 1975), these individuals can still be left-handers, owing to the fact that the model describes a shift in the distribution of skill between the hands that still extends, albeit at low frequency, into the better left-hand skilled range.
Figure 1.
Distribution of lateralization of handedness in proportion of individuals with a certain allele combination, according to three genetic models: (a) Annett's model for hand skill, (b) McManus' model and (c) Klar's model, both for hand preference. 1, homozygote chance; 2, heterozygote; 3, homozygote for right allele. For details see text.
In contrast to the single-locus models, Levy & Nagylaki (1972) proposed a two-loci, four-allele model. One locus encodes for cerebral dominance for speech, the other for either contralateral or ipsilateral hand control relative to the dominant hemisphere. Yeo & Gangestad (1993) proposed that there is little or no direct genetic effect on handedness. A deviation from the moderate right-handed population mean is assumed to be caused by early polygenetic homozygosity causing developmental instability and extreme right- or left-handedness.
(b) Challenges for the models
(i) The twin paradox
There are several general problems concerning the validity of these genetic models. The first emerged from twin studies. To disentangle genetic from environmental factors, many investigators compared monozygotic (MZ) with dizygotic (DZ) twins. MZ twins are more likely to be concordant concerning handedness than DZ twins (see Sicotte et al. (1999) for a meta-analysis), suggesting genetic inheritance. However, between 10 and 25 per cent of MZ twins are still discordant for handedness (Rife 1940; Bryden 1982; Sicotte et al. 1999). Several suggestions were made to fit this MZ twin discordance phenomenon into genetic models. Based on calculations concerning gene frequencies, Klar (1996) expected 18 per cent of the individuals in the population to be lacking the right gene and thus developing direction of lateralization by chance and this could explain the 18.3 per cent discordance in MZ twins found by Rife (1940). This is because lack of the fully penetrant right alleles in Klar's model would induce the individual members of MZ twins to develop handedness at chance independently of each other. However, just as many concordant as discordant twins with this genotype are expected on the basis of change, so that 18 per cent of the genotype would lead to 9 per cent discordant and 9 per cent concordant twins. The theory can thus only account for half of the discordant MZ twins observed in the population. Furthermore, it cannot explain the higher incidence of left-handedness in twins compared with singletons (Sicotte et al. 1999).
Similarly, both McManus (1985a, 1999) and Annett (1972, 1975) proposed that discordant MZ twins could be homozygotic for the chance allele. In addition, due to the additive nature of their models, discordant MZ twins can also be heterozygotic and in Annett's model even homozygotic for the right allele. An addition to Annett's (1978a) model assumes that the right shift caused by the right allele expresses weaker in those who are less mature at birth, and it was proposed that this is the case for twins relative to singletons. This decreased gene expression is assumed to be caused by disturbances of development during a sensitive prenatal period and would explain the high frequency of discordances and the increased incidence of left-handedness in twins compared with singletons (Sicotte et al. 1999, but see Medland et al. 2003). However, Orlebeke et al. (1996) argued that decreased maturation and the supposedly associated reduced expression of the right shift cannot account for increased left-handedness in twins because the first-born twin is heavier and still more often left-handed than the second-born twin.
The model of Levy & Nagylaki (1972) states that individuals with identical genotypes exhibit the same dominant hemisphere for language and the same hand preference, and attributes the prevalence of discordant MZ twins to environmental factors such as pathogenic and mirror-imaging effects (see below).
(ii) Explanations of the twin paradox by environmental factors
Clearly, solely genetic inheritance is unlikely to explain the twin paradox. Proposed environmental explanations for the high proportion of discordant MZ twins include the suggestion that the MZ twinning process itself is pathological (James 1983; Boklage 1987; Levin 1999; Sommer et al. 1999), and the mirror-imaging theory that states that owing to relatively late splitting of the already slightly lateralized embryo, the members of MZ twins represent the ‘right’ and ‘left’ halves of the egg (Newman 1928; Stocks 1933). However, the finding that the incidence of left-handedness is not different between MZ and DZ twins is in contrast with these two hypotheses (Sicotte et al. 1999).
A more viable explanation is that discordant MZ twins are affected by differential environmental factors such as differential perinatal stress that is associated with higher incidences of left-handedness (Soper & Satz 1984; see references in Sicotte et al. (1999) and Hopkins et al. (2000) for chimpanzees). For example, primiparae might be more exposed to birth stress (Orlebeke et al. 1996); twins might influence each other and twin members lay in differential position in the womb (Geschwind & Galaburda 1985), which could affect lateralization in twins.
(iii) Sex differences
The second challenge concerning the genetic models of handedness is that males show higher incidences of left-handedness (11.6%) than females (8.6%) (McManus 2002). A simple autosomal genetic theory may thus not explain this sex difference.
Annett addressed the sex differences in handedness similarly to the way she addressed the twin paradox: the right allele would express weaker in those who are less mature at birth (Annett 1978a; Davis & Annett 1994), which in this case means less in males than females. The parameters of the model thus changes depending on the sex and singleton/twin state of the offspring.
A revision of the McManus' model (1985a) incorporated a novel rare recessive allele located on the X chromosome, which suppresses the autosomal right allele (McManus & Bryden 1992). Higher incidences of left-handedness are then expected in males because males, having only one X chromosome, need only one of this rare recessive allele, whereas females need two. Several other sex-chromosomal linked models have been proposed (Crow 1993, 1995; Jones & Martin 2000). Laval et al. (1998) found evidence for a quantitative trait locus (QTL) on the X chromosome for linkage to relative hand skill. Although this was partly supported by a genome-wide scan, more important linkages to relative hand skill were found on other chromosomes (Francks et al. 2002). Another genome-wide analysis found no evidence for the presence of QTL linked to handedness on the X chromosome (Van Agtmael et al. 2003). These studies suggest that handedness has a genetic component, but that a single-gene model is unlikely and that the genetic factor influencing handedness is most probably multifactorial. However, it is conceivable that these multiple genes may inherit as a single-locus trait. This is for example the case of co-adapted gene complexes that are linked due to their position on the same arm of an inversed part of a chromosome (Kamping & Van Delden 1999). In the case of genes being distributed over several chromosomes, inheritance as a single locus is, however, not conceivable. However, in that case it may account for the random factor postulated to determine lateralization, but not for the dominant allele that would induce right-handedness.
Neurodevelopmental disorders are, just as left-handedness, more common in males. Yeo & Gangestad (1993) proposed that males show higher degrees of polygenetic homozygosity, inducing developmental instability leading to increased left-handedness. They however do not explain the cause of the supposedly increased homozygosity in males.
The incidence of left-handedness is higher when the mother is left-handed and the father is not, than when the father is left-handed and the mother is not (Falek 1959; Porac & Coren 1981; McManus 1991; Annett 1994; Mckeever 2000). This either suggests a form of genomic imprinting or parental effects. Annett addressed this problem based on a Carter (1961) effect. However, the Carter effect can occur when an inherited characteristic is genetically multifactorial, whereas Annett's model is not.
As mentioned earlier, McManus & Bryden (1992) suggested an X-linked recessive gene that can suppress the autosomal right gene. This can explain not only the differences in incidences of left-handedness between males and females, but also this maternal effect. A female carrying two copies of this allele should then produce 100 per cent left-handed sons. Unfortunately, this prediction cannot be tested because the locus of this proposed gene is unknown, if it exists at all.
Klar (1996) did not explain sex differences by genetic factors but attributes them and the maternal effects to environmental factors, such as differential sensitivity to social pressures (see below).
(iv) Explanations of the sex difference and maternal effects by environmental factors
Several environmental factors may explain the higher incidence of left-handedness in males. First, men and women may differ in their sensitivity to social pressures. Females more often report to successfully change hand preference owing to social pressures. Furthermore, both males and females may be more under maternal than paternal social pressures, for example owing to more mother–offspring than father–offspring interactions (Morgan & Corballis 1978; Porac et al. 1986). In addition, as suggested by Falek (1959), left-handed fathers could also be more aware of the disadvantages concerning employment of left-handers than left-handed mothers. This could lead to higher social pressures when the father is left-handed than when the mother is. The offspring of left-handed fathers could thus more often conform to right-handedness. Additionally, it has frequently been suggested that sex differences in lateralization may be due to differential exposure to gonadal steroid hormones (reviewed in Pfannkuche et al. 2009).
(v) Inconsistencies with data
McManus (1985b) showed that a symmetrical bimodal model can describe the handedness skill distribution data at least for some tasks better than the right-shift model of Annett. The model of Klar also faces a problem. One of the predictions of Klar's (1996) model is that right-cerebral dominance for speech is expected in 50 per cent of left-handed individuals (those lacking right alleles). However, several functional magnetic resonance imaging (fMRI) studies in non-pathological left-handers are in conflict with this (Jansen et al. 2007). Furthermore, the prediction that left-handed parents produce 50 per cent left-handed children does not hold (Annett 2008).
The two-loci model of Levy & Nagylaki (1972) is inconsistent with the observation that left-handers tend towards ambilaterality, whereas right-handers show almost complete specialization of the hemispheres (Goodglass & Quadfasel 1954; Subirana 1964). If full expression of the alleles occurs only when a dominant allele is present at both loci, this problem is solved. This is however a post hoc addition to the model and should be tested in a new dataset.
(c) Evidence from animal models
To validate the models and to disentangle between genetic and environmental factors influencing handedness, experimental studies should be performed. Owing to obvious ethical reasons, such studies can only be carried out in non-human animals.
(i) Descriptive evidence
In chimpanzees, handedness was measured by means of a tube task in which peanut butter must be obtained from a tube using one hand. Of the offspring of right-handed mothers 86 per cent were right-handed, but only in second to fifth offspring within a litter in which pregnancies have relatively low developmental instability. In the other offspring, only 46 per cent born to right-handed mothers were right-handed indicating both a heritable and environmental effect (Hopkins et al. 2001). In another study in wild chimpanzees, both maternal-offspring and maternal half-siblings hand preferences were significantly associated and concordance rates in mother–offspring and between maternal half-sibling were higher than chance (Lonsdorf & Hopkins 2005). Annett (2006) suggested that chimpanzees show a genetically determined right shift, although the magnitude of expression was significantly less than that in humans. Although her model may perhaps fit the data, this suggestion is in contrast with her idea that lateralization in handedness has evolved in consort with that for language, since chimpanzees lack the capacity for the latter. Alternatively, the heritable component can be explained by a non-genetical maternal effect (see §3c).
(ii) Experimental evidence
An attempt to selectively breed mice for the direction of pawedness failed, although selective breeding attempts for the degree of pawedness were successful (see Collins (1985) for a review). Variation within the latter strains was still present, suggesting environmental influences. Collins et al. (1993) showed that differences in total heterozygosity did not explain the difference in degree between the strains as was originally proposed by McManus (1992).
We would like to point out that conclusions about the genetic background of a trait based on selective breeding experiments without cross-fostering the offspring should be made with caution. These experiments are not capable of distinguishing between genetic and environmental effects (such as learning). Moreover, in order to rule out any prenatal effects (such as hormones) on lateralization, zygote translocation is necessary.
An artificial selection study in the poeciliid fish Girardinus falcatus on the preference to investigate certain stimuli with either the left or right eye estimated the heritability of degree and direction greater than 0.5 (Bisazza et al. 2000). However, after the first generation, the response to selection ceased. Some potential explanations for the latter finding were suggested in which fish showing the lateralization opposite to the one selected for have an advantage. For example, fish of such opposite lateralization may surprise conspecifics in their approach from the other side, leading to more successful forced copulations or more successful predation (Bisazza et al. 2007).
Hori (1993) investigated the inheritance pattern of lateralization in the fish Perissodus microlepis. These fish eat scales from the flanks of prey fish by attacking them from either the left or right side and have therefore a slightly asymmetrical mouth opening, directed to, respectively, the right or left. He suggested that this ‘mouthedness’ is inherited in a Mendelian fashion with right mouthedness being dominant. This is, however, not consistent with the finding that two left-mouthed parents can produce up to 25 per cent right-mouthed offspring. Later, Hori et al. (2007) adjusted the explanation by suggesting that the right-mouthed allele is lethal when homozygous. However, the data of Hori (1993) suggested that homozygous right-mouthed fish are present in the population. The inheritance pattern of this trait thus remains unclear.
(d) In conclusion
Although several elegant genetic models for lateralization of handedness and language fit well the majority of the distribution and inheritance data by assuming certain rules for genetic inheritance, they require several ad hoc additions for explaining deviations from the main pattern. These additions are not always fully supported by independent data. This may either suggest that the specific deviations, such as the twin, sex and maternal effects, may be best explained by environmental factors, for which indeed some suggestions have been made in the literature; or it may even suggest that the basic assumptions of the models are not correct, as has been discussed earlier. The latter is supported by the fact that the few genome scans performed concerning handedness could not find evidence for a simple genetic model, but suggest a more complex interplay between different genes involved.
In any case, the models do not rule out an important role for environmental influences on the development of lateralization. Interestingly, models such as those from Annett (1972, 1985) and Klar (1996) explicitly need environmental factors to fit the observed incidences of left-handedness.
Few attempts to identify the potential genetic background of handedness in non-human animals have been performed. So far, the results are inconsistent with each other and with the human models proposed, although Annett (2006) suggested some resemblance between humans and chimpanzees in the genetic inheritance of hand-use lateralization. No genetic models for lateralization in animals have been built and human models have hardly been tested in animals. More animal studies are crucially needed to investigate the inheritance of laterality in animals. This could shed light on its evolution and generate hypotheses for its inheritance in humans.
3. Potential environmental factors
Section 2 indicates that there is ample opportunity for environmental factors to affect the development of behavioural lateralization. In this section, we will discuss these factors in more detail, focusing on the potential effects of social modulation such as social pressures and parental effects (including cradling), as well as asymmetric input of stimuli. Additionally, the organizational and activational effects of steroid hormones have been suggested to be relevant for lateralization. This topic will be discussed, together with sex differences in lateralization, in a separate paper where we present the results of several meta-analyses (Pfannkuche et al. 2009).
We start with a short description of development of behavioural lateralization in order to establish when, and to what extent, it gradually develops. This may indicate to what degree and in which stage in development there is scope for environmental effects to act. We will not focus on pathological development. It is well known that the incidence of left-handedness is positively related to behavioural disorders, birth stress and low birth weight (Bakan et al. 1973; Coren 1993 for a review), and this is reviewed in another contribution to this issue (Llaurens et al. 2009).
(a) Early development of handedness
Human foetuses prefer to use the right hand for thumb sucking already in the third trimester independent of lying position in the womb (Hepper et al. 1991). Thumb-sucking behaviour, but no other prenatal hand–mouth contacts (de Vries et al. 2001), is a good predictor for handedness later in life (Hepper et al. 2005). Similarly, prenatal head position shortly before birth correlates with the preferred head position of neonates in a supine position, which again correlates with handedness in reaching tasks 12–74 weeks post-partum (see §3b(ii)). Although these data suggest that predispositions for handedness are already present early in ontogeny, they do not exclude a role for environmental factors affecting lateralization later in life. In fact, prenatal influences may be very important (see below and, e.g. Pfannkuche et al. 2009). In addition, during early childhood, handedness still shows considerable fluctuations (Gesell & Ames 1947; Goldfield & Michel 1986; Corbetta et al. 2006; Michel et al. 2006). Not until the age of 4 years right-handed behaviour predominates and unilateral hand preference is well established at the age of 9 (Gesell & Ames 1947). Therefore, the data suggest that although predispositions for lateralization are present already early in ontogeny, handedness is still open to environmental influences later in life, much as early predispositions for motor patterns (courtship postures and calls) and cognition (imprinting on the mother) in birds can still be modified in later life (Johnson et al. 1985; Groothuis 1993).
(b) Environmental factors: asymmetric input of stimuli
(i) Head position in humans
A few weeks before birth, the foetus' head position becomes fixed in utero. Of the 97 per cent of foetuses that lie in a cephalic position, two-thirds lie with their right ear and one-third with the left ear facing out (Michel & Goodwin 1979; Previc 1991 and references therein). This position correlates strongly with the head position of the neonates that lie in a supine position (Michel & Goodwin 1979). The supine head orientation affects the experience with the right and left hand. Previc (1991) has argued that this 2 : 1 ratio is more characteristic for many behavioural asymmetries in human and non-human populations than the 9 : 1 ratio typical for human handedness. He proposes that these asymmetries originate from an asymmetrical prenatal development of the ear and labyrinth. Speech is then lateralized through a slight right ear advantage in the mid-frequency sound range. This advantage is derived from an asymmetrical craniofacial development. Vestibular lateralization, which is linked to motor behaviour, can be traced back to the asymmetrical head position of the foetus during the final trimester. This asymmetry would come about through the differential experience of the left and right vestibules in the final trimester caused by motoric movements of the mother, perhaps creating a pathway for maternal effects discussed earlier in §2.
Most (70–80%) neonates prefer to turn their head to the right side when they are in a supine position (Michel & Goodwin 1979; Michel 1981; Konishi et al. 1986; Previc 1991; Ronnqvist et al. 1998; Ronnqvist & Hopkins 2000; Damerose & Vauclair 2002). This preference appears at the second day of life, at which time they are also more reactive to sounds on the right-hand side (Turkewitz et al. 1966). This tendency diminishes in the course of development. The supposed effects on functional motor lateralities have therefore been argued to be only transient (Konishi et al. 1986). However, the amount of spontaneous visual experience with each hand, which is dominated by head position, predicts which hand predominated in visually elicited reaching at 12 weeks (Coryell & Michel 1978). Moreover, as already mentioned, head orientation in a supine position correlates with handedness during reaching in the period 12–74 weeks post-partum (Kuo & Shen 1937). Inducing differential experience with hands during early development has been a worldwide natural experiment. Across the globe, there have been large-scale changes in placing babies in a supine or prone position in their cribs, due to change in medical advice. As mentioned, in the supine position, there is a natural bias towards right-hand use, whereas in the prone position there is no expression of preference. This is because of the parental strategy of alternating the baby's head to the left and right in order to avoid asymmetrical skull development, and because of the baby's inability to change the head position in the first months by itself. Interestingly, there was an increase in non-right-handed toddlers (at 18 months of age) that were reared in the prone position (Konishi et al. 1987). This suggests that head position is causative to handedness. We are currently conducting a study in The Netherlands to see whether we can replicate this finding.
(ii) Head position in other animal species
Except for birds, it is unknown whether head position is related to lateralization of brain and behaviour in non-human species. Owing to the asymmetrical position of the avian head in the egg, one eye is positioned against the body, whereas the other lies against the eggshell. Light can penetrate the shell and induce brain lateralization (see below). However, one should realize that the indirect effect of head position on lateralization via its effect on light input has not been disentangled from a direct effect of head position, irrespective of light exposure. Although avian models are often used to study the development of lateralization, quantitative data support the general idea that bird embryos are folded in the egg in such a way that almost all of them receive light with the right eye due to their head position (Oppenheim 1973) are surprisingly scarce, and some data suggest much more variation (Riedstra 2003). If the variation of head position is substantial, this may severely influence the outcome of experiments manipulating embryonic light exposure. We found that fMRI techniques can be successfully used to identify the turning position in eggs without exposing them to light (B. Riedstra 2007, personal observation).
(iii) Asymmetric light input in birds
Many bird species (galliformes, pigeons, parrots, raptors and songbirds) show behavioural lateralization in visually guided behaviours (e.g. Andrew & Brennan 1983; ten Cate et al. 1990; ten Cate 1991; Rogers 1996; Alonso 1998; Manns & Gunturkun 1999; Bobbo et al. 2002; Templeton & Gonzalez 2004), motor patterns (Rogers & Workman 1993; Goller & Suthers 1995; Csermely 2004) and cognitive functions (Nottebohm 1970, 1971; Clayton & Krebs 1994, 1995; Floody & Arnold 1997; Gagliardo et al. 2001; Nottelmann et al. 2002). Lateralization of visually guided behaviours is influenced by asymmetrical light exposure in the period shortly before hatching. Light reaching the eye through the eggshell induces growth of the visual projections from the exposed eye to the contralateral hemisphere and induces functional lateralization (Rogers 1996). Hemispheric control of attack and copulation becomes dominant in the hemisphere contralateral of the light-exposed eye, both when exposing the naturally exposed eye or by experimentally exposing the normally occluded eye to light (Rogers 1990). Chicks receiving no light also become lateralized but the direction of lateralization is unpredictable (Rogers 1982). In addition, dark-incubated chicks become less strongly lateralized and have poorer performances in dual tasks (Dharmaretnam & Rogers 2005). Unfortunately, further studies addressing the extent and nature of lateralization in dark-incubated chicks are lacking, although these could reveal to what extent other factors than light guide the development of lateralization.
It is not our intention here to review the literature on light-induced lateralization in birds since excellent reviews on this topic are available (e.g. Rogers 1996). However, we stress that there is no evidence showing that asymmetrical light exposure during the last phase of incubation is really the default situation in nature. Only one study detailed the amount of light exposure to eggs during the incubation period and concluded that this was sufficient to induce lateralization (Buschmann et al. 2006). As there is large variation in eggshell properties, nest sites determining light availability and incubation patterns among avian species, the generality is questionable. Moreover, the adaptive advantage of lateralization has recently been questioned too (Hirnstein et al. 2008). In addition, only one study has addressed the question of whether manipulation of light exposure during incubation has consequences in adulthood (Manns & Gunturkun 1999). This is very relevant as the effect of early light exposure on asymmetrical visual pathways seem to diminish with age in the chicken (Rogers 1995). Since we are here concerned with the mechanisms of development of lateralization, and not its functional relevance, this will not be a topic of this paper.
Finally, light has pleiotropic effects that may confound experiments that manipulate embryonic light exposure. Prenatal light exposure also increases growth rate and hatching time but reduces hatchling weight (Adam & Dimond 1971; Evans & Evans 1999; Shafey & Al-Mohsen 2002; Shafey 2004). If these factors affect behavioural and brain lateralization, as birth weight and perinatal stress in humans, then light may affect lateralization via other pathways than asymmetrical light input only.
(iv) Cradling in humans
Right-handed and dextro-cordius mothers prefer to hold infants on the left arm (left-handed females have not been reported for right-side-holding biases, but no sufficient data exist; Donnot 2007), whereas males have no preference (Damerose & Vauclair 2002). Cradling by mothers thus induces asymmetrical auditive and visual input, head and arm position, potentially influencing development of lateralization. However, left-handed cradling may actually restrict right-arm movements of the baby and thereby perhaps development of right-handedness. Furthermore, although there is some evidence that the emotional hemispheric specialization of the holder predicts holding bias in left-handed students, but not in left-handed mothers (Donnot 2007), the effect on the baby's lateralization is not yet known. There is also some evidence that the baby's head-turning preference modulates the side preference of adult handling, but not the other way around (Bundy 1979). In conclusion, evidence for an influence on lateralization of the baby is lacking. Longitudinal studies on children until their hand preference are stable in relation to cradling experience, for example in societies that differ in cradling behaviour, may be of help. This may perhaps also explain part of the difference in the frequency of left-handedness observed among societies (see also §3e).
(c) Environmental factors: adoption in humans and animals
In an attempt to disentangle between genetic and environmental factors determining handedness, investigators have focused on adoption studies. Surprisingly, parent–offspring correlations concerning strength and direction of hand preference were absent in both adopted and non-adopted children (Rice et al. 1984), perhaps due to the very young age of the children investigated (12–24 months). Two other studies showed different results. Hicks & Kinsbourne (1976) found that hand preferences of students significantly correlated with the writing hand of their biological parent, but not with that of their step-parent. Although the authors statistically controlled for the time spent living with the step-parent, it is most likely that the hand preference was already established in the students long before the step-parent could influence this preference, since the mean age of the students when the step-parent moved in was approximately 13 years of age (s.d.=3.12). However, a similar outcome was found in a study in which all adopted children were taken into the participating families before the age of 1 (Carter-Saltzman 1980). However, the possibility that lateralization and handedness are determined before that age, although not yet fully expressed, is still conceivable (see §§ 2a and 3b(i)(iv) and Pfannkuche et al. 2009). To our knowledge, only one cross-fostering study on handedness, measured by means of a tube task with peanut butter (see above), has been conducted in non-human animals. In cross-fostered chimpanzee siblings, the concordance rate in hand preference was not greater than chance, whereas this was the case for siblings that were reared together, strongly suggesting that the underlying mechanisms controlling handedness are heritable, but not genetic (Hopkins 1999). In conclusion, early cross-fostering studies suggest a strong heritable component, and the chimpanzee studies indicate that this may be a non-genetic effect.
(d) Changes with age
In humans, cross-sectional studies reveal that right-handedness increases with age (Fleminger et al. 1977; Smart et al. 1980; Brackenridge 1981; Brito et al. 1985; Beukelaar & Kroonenberg 1986; Lansky et al. 1988; Dellatolas et al. 1991; Gilbert & Wysocki 1992; Iwasaki et al. 1995; De Agostini et al. 1997; Ellis et al. 1998; McManus 2002). Several hypotheses have been postulated to explain this phenomenon. (i) Since left-handedness has been correlated to lower survival, this might result in the decrease in the incidence of left-handedness among elderly people (Halpern & Coren 1988; Coren 1989; Coren & Halpern 1991). (ii) Social pressures against left-handedness over the years declined, so that younger people are less restricted and therefore show higher incidences of left-handedness (Hildreth 1949; Levy 1974; Brackenridge 1981; Leiber & Axelrod 1981 and references in Harris 1990). Furthermore, with increasing age, the number of social contacts increase, which may enhance the probability to switch towards right-handedness. (iii) Humans live in a right-biased world. Tools are made for right-handed individuals and this will in time cause a shift towards dextrality in left-handed individuals and strengthens right-handedness in right-handers (Porac & Coren 1981). (iv) Cerebral dominance development is a continuous process that evolves throughout life and causes the increase in right-handedness with age (Brown & Jaffe 1975; Fleminger et al. 1977). (v) An information bias in handedness questionnaires has been proposed, resulting in a change in the categorization of handedness (Fleminger et al. 1977). This does not seem likely as most studies investigating the effect of age on handedness are cohort studies. To distinguish between these hypotheses, longitudinal studies that investigate the development of lateralization within the individuals are clearly needed.
(e) Environmental factors: social pressures
(i) Evidence in humans
Although right-handers outnumber left-handers in all societies studied, differences in the percentages of right-handedness have been observed among different societies: sinistrality being, in general, higher in Western societies than in other societies (Iwasaki (2000) and references therein). These differences could be caused either by environmental factors such as increased social pressures in some societies, or by a decreased number of the proposed right allele in the gene pool of certain populations. McManus (2002) hypothesized that it was possible to disentangle between these genetic and environmental factors by investigating how strongly handedness runs in families. He assumed that if social pressures to be right-handed are strong, left-handedness will run less strongly in families. This assumption is not necessarily right as differences in social pressures may not be equal for all individuals and vary between families. Porac et al. (1986) found some evidence for this. He investigated social pressures within families by assessing the amount of attempts to switch handedness: males from right-handed parents were more likely to switch from left- to right-hand use than males from one or two left-handed parents. McManus' conclusion that the decreased incidence of left-handedness in non-Western populations is due to a decreased incidence of the right allele might be false as it can also be explained by differential social pressures between families. The hypothesis that social pressures can decrease the incidence of left-handedness is further strengthened by the finding of Dawson (1977) who found that more conforming agriculturalists measured by means of the Asch Conformity Test show low incidences of left-handedness (0.6–3.4%), whereas permissive, non-conforming populations show extensively higher incidences of left-handedness (11.3–10.5%).
(ii) Evidence for other animal species
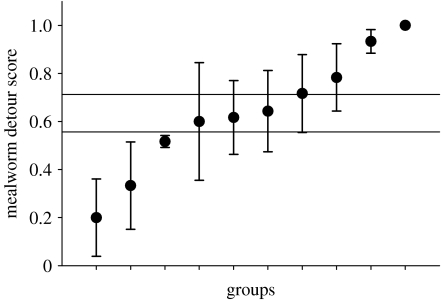
To our knowledge, there are no studies that have tested the possibility of social modulation affecting behavioural lateralization in non-human animals. However, we recently found some evidence for this possibility. Eggs of laying hens (Gallus gallus domesticus) were incubated under standard conditions. The chicks were housed in 10 groups of 6 (±1) individuals in the same room. At day 4–6, post-hatching behavioural lateralization was assessed by detour tests. Chicks had to detour a see-through barrier to reach either a group of unknown conspecifics or a mealworm. The side used to detour the barrier was scored in two bouts of five consecutive trials on two consecutive days for both stimuli. Preferred eye use for each stimulus, determined by hemispheric organization, is thought to determine the side of rounding the barrier (Vallortigara et al. 1999). Individuals showed consistent choices between tests (r2=0.69, p<0.0001, n=58), with most individuals preferring to turn right in both tests. This suggests that approaching food and unknown conspecifics are functionally located in the same, predominantly right hemisphere. Interestingly, the variation in lateralization was smaller within groups than among groups (figure 2; F=12.66, p<0.001). This is the first evidence suggesting that lateralization of visually guided behaviours can be modulated by post-hatching social interactions. This could ensure the hypothesized benefits of a group bias in lateralization, as suggested by Vallortigara & Rogers (2005). However, since the experiment was not designed for testing this hypothesis, this post hoc finding is currently being replicated.
Figure 2.
Detour scores: each circle represents the group mean (+s.e.) of six chicks, which round a barrier to reach a mealworm. Variation in lateralization was smaller within than between groups (F=12.66, p<0.001) indicating that lateralization was modulated by post-hatching social interactions.
Furthermore, Collins (1968) conducted an experiment in which the effects of social pressures were altered. He found that in a non-biased environment (no pressure) in which food could be obtained with either paw equally well, mice had a side preference, but no population bias was observed. When the feeding tube was placed against the right wall in such a way that obtaining food was easier using the right than the left paw (mimicking the right-biased world of humans), 90 per cent of the mice showed a right paw preference (Collins 1975). This result was attributed to a change in paw use in weakly left-lateralized individuals. If this is the case, the direction and degree of handedness are not independent factors. Collins suggested that right-handedness might work similarly in humans. Collins et al. (1993) concluded that the observed differences found in the heritability between degree and direction in humans and mice should not necessarily lead to different underlying mechanisms. In conclusion, evidence exists for social modulation of handedness in both humans and other animal species.
4. Discussion
The long-standing debate about the question of whether lateralization of brain and behaviour is caused by genes or environment actually focuses on a wrong question. Modern developmental biology has recognized for decades that the phenotype develops under the continuous interaction between genetic and environmental influences and that both are indispensable for development. Moreover, in the end product of this interactive developmental process, both factors are impossible to disentangle. Therefore, by demonstrating the influence of either genetic or environmental components, we cannot conclude anything conclusive about the contribution of the other component on the developmental process. However, correlative and experimental studies can demonstrate which factors are important, and how they interact. Unfortunately, gene–environment interactions have not been explicitly studied, but for instance the difference in lateralization between light- and dark-reared birds in which the latter still show some degree of it (Rogers 1995) does suggest such an interaction. Moreover, owing to the historical focus on humans, descriptive studies outnumber experimental studies by far. We hope that this review will stimulate researchers to bring the field more into balance.
It has been questioned to what extent lateralization in humans and other vertebrates may be comparable. We agree that it is likely that humans may have species-specific adaptations in their lateralized behaviour. This may explain the strong human lateralization in handedness due to selection on efficient tool use or language (Corballis 2003). Nevertheless, we strongly believe that lateralization of brain and behaviour, being such a fundamental aspect of the organization in vertebrates, must share common principles for humans and other vertebrates, similarly to the blueprint for vertebrate skeleton, physiology, brain and behaviour.
Evidence for a genetic basis of lateralization in humans is mainly based on demographic and heritability studies of handedness, and the explanatory power of genetic models. As argued earlier, the evidence from demographic and heritability studies does not disentangle genetic from environmental factors such as parental effects, and even early adoption studies cannot circumvent prenatal maternal effects. Evidence from the modelling approach is not yet fully convincing either. Despite their elegance and clever design, the models have limited explanatory power and are not backed up by the data from human genome scans, which suggest a multi-genetic control of human lateralization. Unfortunately, data from animal experiments concerning the genetics of lateralization are also inconclusive. Although the few selection experiments in animals give some support for genomic heritability, the results are ambiguous and the studies not always properly conducted.
Although some data suggest exciting possibilities, evidence for environmental influences on lateralization is ambiguous too. Descriptive data that show changes with age are not conclusive for environmental effects since they may be genetically encoded. Moreover, in order to assess developmental principles of lateralization, longitudinal studies are needed. In humans, the available data suggest that although predispositions for handedness may be present already prenatally and predictive for later lateralization, handedness can to some extend still change in later life. The correlation between early developmental disorders and left-handedness suggests a role for early environmental modulation, but does not tell us necessarily much about the environmental effects on undisturbed development.
The possibility that in humans, left-handers are in fact a heterogeneous group of pathological and ‘normal’ left-handers complicates research to a large extent. Actually, the genetic models suggest that also the right-handers are a heterogeneous group consisting of both genetically right- and left-handers. Interpretation is further complicated by the use of different criteria to categorize handedness. Finally, more attention should be paid to other forms of behavioural lateralization, which may not always correlate with handedness, and may be more similar to lateralization indices in animals. Unfortunately, in animals even less is known about typical development and to what extent early manipulations still exert their effect in adulthood. Such long-term studies take time, but are very relevant for further progress in the field.
Five lines of evidence suggest a role for environmental modulation of lateralized behaviour. First, the finding that rearing position of the neonate seems to affect handedness, based on a natural experiment whereby mothers were instructed differently to keep their babies in a supine or prone position (Konishi et al. 1987). It opens an exciting perspective, although we cannot rule out a confounding effect of time here and the study needs replication. Second, the study of cross-fostered chimpanzees (Hopkins 1999) indicated strong rearing effects, although this is in contrast with a study of early cross-fostering in humans (Carter-Saltzman 1980). Third, there is evidence that prenatal exposure to steroid hormones affects lateralization in humans (Pfannkuche et al. 2009). Fourth, our data on social modulation in the domestic chick warrant further research in this direction. Fifth, the effect of asymmetrical light input caused by the asymmetrical position of the head in bird embryos has now become a classical example of how early environmental factors can influence lateralization. This is consistent with the suggestion that pre- and post-natal head position may affect lateralization by asymmetrical perception in humans. Nevertheless, further studies documenting head position and light input in bird eggs and their long-term effects are necessary for interpreting the findings from a functional perspective. Furthermore, by manipulating head position together with light input, the influence of both factors can be disentangled.
In conclusion, there is evidence for both genes and environment to affect the development of behavioural lateralization, but evidence for both and especially their interaction is surprisingly incomplete. With the identification of the human genome, and the use of animal models, we believe that substantial progress can be made in the near future. For example, by setting up selection lines for differences in strength or direction in lateralization and exposing them to different environmental influences such as prenatal hormone exposure, asymmetrical stimulus input, or exposure to conspecifics that are lateralized in only one direction, gene–environment interactions can be studied experimentally.
Acknowledgments
All experiments were carried out under license of the animal experiments committee of the University of Groningen (DECnr 4519).We thank Reint Geuze and two anonymous referees for their helpful suggestions that improved the manuscript, and Mirre Simons and Gert Stulp for their help with the chick experiment on social modulation. The paper was written with the help of an EU grant (EDCBNL network) to T.G.G.G.
Footnotes
One contribution of 14 to a Theme Issue ‘Mechanisms and functions of brain and behavioural asymmetries’.
References
- Adam J.H., Dimond S.J. Influence of light on the time of hatching in the domestic chick. Anim. Behav. 1971;19:226–229. doi:10.1016/S0003-3472(71)80002-7 [Google Scholar]
- Alonso Y. Lateralization of visual guided behaviour during feeding in the zebra finches (Taeniopygia guttata) Behav. Process. 1998;43:257–263. doi: 10.1016/s0376-6357(98)00015-1. doi:10.1016/S0376-6357(98)00015-1 [DOI] [PubMed] [Google Scholar]
- Andrew R.J., Brennan A. The lateralization of fear behaviour in the male domestic chick: a developmental study. Anim. Behav. 1983;31:1166–1167. doi:10.1016/S0003-3472(83)80023-2 [Google Scholar]
- Annett M. The distribution of manual asymmetry. Br. J. Psychol. 1972;63:343–358. doi: 10.1111/j.2044-8295.1972.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Annett M. Hand preference and the laterality of cerebral speech. Cortex. 1975;11:305–328. doi: 10.1016/s0010-9452(75)80024-4. [DOI] [PubMed] [Google Scholar]
- Annett M. Lanchester Polytechnic; Coventry, UK: 1978a. A single gene explanation of right and left handedness and brainedness. [Google Scholar]
- Annett M. Genetic and non-genetic influences on handedness. Behav. Genet. 1978b;8:227–249. doi: 10.1007/BF01072826. doi:10.1007/BF01072826 [DOI] [PubMed] [Google Scholar]
- Annett M. Erlbaum; London, UK: 1985. Left, right, hand and brain: the right shift theory. [Google Scholar]
- Annett M. Handedness as a continuous variable with dextral shift: sex, generation, and family handedness in subgroups of left-handers and right-handers. Behav. Genet. 1994;24:51–63. doi: 10.1007/BF01067928. doi:10.1007/BF01067928 [DOI] [PubMed] [Google Scholar]
- Annett M. Psychology Press; Hove, UK: 2002. Handedness and brain asymmetry: the right shift theory. [Google Scholar]
- Annett M. The distribution of handedness in chimpanzees: estimating right shift in Hopkins' sample. Laterality. 2006;11:101–109. doi: 10.1080/13576500500376500. [DOI] [PubMed] [Google Scholar]
- Annett M. Tests of the right shift genetic model for two new samples of family handedness and for the data of McKeever (2000) Laterality. 2008;13:105–123. doi: 10.1080/13576500701433522. [DOI] [PubMed] [Google Scholar]
- Bakan P., Dibb G., Reed P. Handedness and birth stress. Neuropsychologia. 1973;11:363–366. doi: 10.1016/0028-3932(73)90050-x. doi:10.1016/0028-3932(73)90050-X [DOI] [PubMed] [Google Scholar]
- Beukelaar L.J., Kroonenberg P.M. Changes over time in the relationship between hand preference and writing hand among left-handers. Neuropsychologia. 1986;24:301–303. doi: 10.1016/0028-3932(86)90066-7. doi:10.1016/0028-3932(86)90066-7 [DOI] [PubMed] [Google Scholar]
- Bisazza A., Facchin L., Vallortigara G. Heritability of lateralization in fish: concordance of right-left asymmetry between parents and offspring. Neuropsychologia. 2000;38:907–912. doi: 10.1016/s0028-3932(00)00018-x. doi:10.1016/S0028-3932(00)00018-X [DOI] [PubMed] [Google Scholar]
- Bisazza A., Dadda M., Facchin L., Vigo F. Artificial selection on laterality in the teleost fish Girardinus falcatus. Behav. Brain Res. 2007;178:29–38. doi: 10.1016/j.bbr.2006.11.043. doi:10.1016/j.bbr.2006.11.043 [DOI] [PubMed] [Google Scholar]
- Bishop D.V.M. Individual differences in handedness and specific speech and language impairment: evidence against a genetic link. Behav. Genet. 2001;31:339–351. doi: 10.1023/a:1012239617367. doi:10.1023/A:1012239617367 [DOI] [PubMed] [Google Scholar]
- Bobbo D., Galvani F., Macetti G.G., Vallortigara G. Light exposure of the chick embryo influences monocular sleep. Behav. Brain Res. 2002;134:447–466. doi: 10.1016/s0166-4328(02)00059-1. doi:10.1016/S0166-4328(02)00059-1 [DOI] [PubMed] [Google Scholar]
- Boklage C.E. Twinning, nonrighthandedness, and fusion malformations: evidence for heritable causal elements held in common. Am. J. Med. Genet. B. 1987;28:67–84. doi: 10.1002/ajmg.1320280111. [DOI] [PubMed] [Google Scholar]
- Brackenridge C.J. Secular variation in handedness over 90 years. Neuropsychologia. 1981;19:459–462. doi: 10.1016/0028-3932(81)90076-2. doi:10.1016/0028-3932(81)90076-2 [DOI] [PubMed] [Google Scholar]
- Brito G.N.O., Brito L.S.O., Paumgartten F.J.R. Effect of age on handedness in Brazilian adults is sex-dependent. Percept. Motor Skills. 1985;61:829–830. [Google Scholar]
- Brown J.W., Jaffe J. Hypothesis on cerebral dominance. Neuropsychologia. 1975;13:107–110. doi: 10.1016/0028-3932(75)90054-8. doi:10.1016/0028-3932(75)90054-8 [DOI] [PubMed] [Google Scholar]
- Bryden M.P. Academic Press; New York, NY: 1982. Laterality, functional asymmetry in the intact brain. [Google Scholar]
- Bundy R.S. Effects of infant head position on sides preference in adult handling. Infant Behav. Dev. 1979;2:355–358. doi:10.1016/S0163-6383(79)80045-4 [Google Scholar]
- Buschmann J.U.F., Manns M., Gunturkun O. “Let there be light!” Pigeon eggs are regularly exposed to light during breeding. Behav. Process. 2006;73:62–67. doi: 10.1016/j.beproc.2006.03.012. doi:10.1016/j.beproc.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Carter C.O. The inheritance of congenital pyloric stenosis. Br. Med. Bull. 1961;17:251–253. doi: 10.1093/oxfordjournals.bmb.a069918. [DOI] [PubMed] [Google Scholar]
- Carter-Saltzman L. Biological and sociocultural effects on handedness: comparison between biological and adoptive families. Science. 1980;209:1263–1265. doi: 10.1126/science.7403887. doi:10.1126/science.7403887 [DOI] [PubMed] [Google Scholar]
- Clayton N.S., Krebs J.R. Memory for spatial and object related cues in food-storing and nonstoring birds. J. Comp. Physiol. A. 1994;174:371–379. [Google Scholar]
- Clayton N.S., Krebs J.R. Lateralization in memory and the avian hippocampus in food-storing birds. In: Alleva E., Fasolo A., Lipp H.-P., Nadel L., Ricceri L., editors. Behavioural brain research in naturalistic and semi-naturalistic settings. Kluwer Academic; Dordrecht, The Netherlands: 1995. pp. 139–157. [Google Scholar]
- Collins R.L. On inheritance of handedness. I. Laterality in inbred mice. J. Hered. 1968;59:9–12. doi: 10.1093/oxfordjournals.jhered.a107656. [DOI] [PubMed] [Google Scholar]
- Collins R.L. When left-handed mice live in right-handed worlds. Science. 1975;187:181–184. doi: 10.1126/science.1111097. doi:10.1126/science.1111097 [DOI] [PubMed] [Google Scholar]
- Collins R.L. On the inheritance of direction and degree of asymmetry. In: Glick S., editor. Cerebral lateralization in nonhuman species. Academic Press; Orlando, FL: 1985. pp. 41–71. [Google Scholar]
- Collins R.L., Sargent E.E., Neumann P.E. Genetic and behavioral tests of the McManus hypothesis relating response to selection for lateralization of handedness in mice to degree of heterozygosity. Behav. Genet. 1993;23:413–421. doi: 10.1007/BF01067444. doi:10.1007/BF01067444 [DOI] [PubMed] [Google Scholar]
- Corballis M.C. From mouth to hand: gesture, speech, and the evolution of right-handedness. Behav. Brain Sci. 2003;26:199–260. doi: 10.1017/s0140525x03000062. [DOI] [PubMed] [Google Scholar]
- Corbetta D., Williams J., Snapp-Childs W. Plasticity in the development of handedness: evidence from normal development and early asymmetric brain injury. Dev. Psychobiol. 2006;48:460–471. doi: 10.1002/dev.20164. doi:10.1002/dev.20164 [DOI] [PubMed] [Google Scholar]
- Coren S. Left-handedness and accident-related injury risk. Am. J. Public Health. 1989;79:1040–1041. doi: 10.2105/ajph.79.8.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coren S. Vintage Books; New York, NY: 1993. The left-hander syndrome: the causes and consequences of left-handedness. [Google Scholar]
- Coren S., Halpern D.F. Left-handedness: a marker for decreased survival fitness. Psychol. Bull. 1991;109:90–106. doi: 10.1037/0033-2909.109.1.90. doi:10.1037/0033-2909.109.1.90 [DOI] [PubMed] [Google Scholar]
- Coryell J., Michel G.F. How supine postural preferences of infants can contribute towards the development of handedness. Infant Behav. Dev. 1978;1:245–257. doi:10.1016/S0163-6383(78)80036-8 [Google Scholar]
- Crow T.J. Sexual selection, Machiavellian intelligence, and the origins of psychosis. Lancet. 1993;342:594–598. doi: 10.1016/0140-6736(93)91415-i. doi:10.1016/0140-6736(93)91415-I [DOI] [PubMed] [Google Scholar]
- Crow T.J. A Darwinian approach to the origins of psychosis. Br. J. Psychiatry. 1995;167:12–25. doi: 10.1192/bjp.167.1.12. doi:10.1192/bjp.167.1.12 [DOI] [PubMed] [Google Scholar]
- Csermely D. Lateralisation in birds of prey: adaptive and phylogenetic considerations. Behav. Process. 2004;67:511–520. doi: 10.1016/j.beproc.2004.08.008. doi:10.1016/j.beproc.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Damerose E., Vauclair J. Posture and laterality in human and non-human primates: asymmetries in maternal handling and the infant's early motor asymmetries. In: Rogers L.J., Andrew M., editors. Comparative vertebrate lateralization. Cambridge University Press; Cambridge, UK: 2002. pp. 306–362. [Google Scholar]
- Davis A., Annett M. Handedness as a function of twinning, age and sex. Cortex. 1994;30:105–111. doi: 10.1016/s0010-9452(13)80326-7. [DOI] [PubMed] [Google Scholar]
- Dawson J.L.M.B. An anthropological perspective on the evolution and lateralization of the brain. Ann. NY Acad. Sci. 1977;299:424–447. doi: 10.1111/j.1749-6632.1977.tb41927.x. doi:10.1111/j.1749-6632.1977.tb41927.x [DOI] [PubMed] [Google Scholar]
- De Agostini M., Khamis A.H., Ahui A.M., Dellatolas G. Environmental influences in hand preference: an African point of view. Brain Cognit. 1997;35:151–167. doi: 10.1006/brcg.1997.0935. doi:10.1006/brcg.1997.0935 [DOI] [PubMed] [Google Scholar]
- Dellatolas G., Tubert P., Castresana A., Mesbah M., Giallonardo T., Lazaratou H., Lellouch J. Age and cohort effects in adult handedness. Neuropsychologia. 1991;29:225–261. doi: 10.1016/0028-3932(91)90086-n. doi:10.1016/0028-3932(91)90086-N [DOI] [PubMed] [Google Scholar]
- Denny K., O'Sullivan V. The economic consequences of being left-handed: some sinister results. J. Hum. Resour. 2007;42:353–374. [Google Scholar]
- de Vries J.I.P., Wimmers R.H., Ververs I.A.P., Hopkins B., Savelsbergh G.J.P., Van Geijn H.P. Fetal handedness and head position preference: a developmental study. Dev. Psychobiol. 2001;39:171–178. doi: 10.1002/dev.1042. doi:10.1002/dev.1042 [DOI] [PubMed] [Google Scholar]
- Dharmaretnam M., Rogers L.J. Hemispheric specialization and dual processing in strongly versus weakly lateralized chicks. Behav. Brain Res. 2005;162:62–70. doi: 10.1016/j.bbr.2005.03.012. doi:10.1016/j.bbr.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Donnot J. Lateralisation of emotion predicts infant-holding bias in left-handed students, but not in left-handed mothers. Laterality. 2007;12:216–217. doi: 10.1080/13576500601182385. [DOI] [PubMed] [Google Scholar]
- Ellis S.J., Ellis P.J., Marshall E., Windridge C., Jones S. Is forced dextrality an explanation for the fall in the prevalence of sinistrality with age? A study in northern England. J. Epidemiol. Commun. Health. 1998;52:41–44. doi: 10.1136/jech.52.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.S., Evans L. Chicken food calls are functionally referential. Anim. Behav. 1999;58:307–319. doi: 10.1006/anbe.1999.1143. doi:10.1006/anbe.1999.1143 [DOI] [PubMed] [Google Scholar]
- Falek A. Handedness: a family study. Am. J. Hum. Genet. 1959;11:52–62. [PMC free article] [PubMed] [Google Scholar]
- Fleminger J.J., Dalton R., Standage K.F. Age as a factor in handedness of adults. Neuropsychologia. 1977;15:471–473. doi: 10.1016/0028-3932(77)90101-4. doi:10.1016/0028-3932(77)90101-4 [DOI] [PubMed] [Google Scholar]
- Floody O.R., Arnold A.P. Song lateralization in the zebra finch. Horm. Behav. 1997;31:25–34. doi: 10.1006/hbeh.1997.1368. doi:10.1006/hbeh.1997.1368 [DOI] [PubMed] [Google Scholar]
- Francks C., Fisher S.E., MacPhie I.L., Richardson A.J., Marlow A.J., Stein J.F., Monaco A.P. A genomewide linkage screen for relative hand skill in sibling pairs. Am. J. Hum. Genet. 2002;70:800–805. doi: 10.1086/339249. doi:10.1086/339249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardo A., Ioale P., Odetti F., Bingman V.P., Siegel J.J., Vallortigara G. Hippocampus and homing in pigeons: left and right hemispheric differences in navigational map learning. Eur. J. Neurosci. 2001;13:1617–1624. doi: 10.1046/j.0953-816x.2001.01522.x. doi:10.1046/j.0953-816x.2001.01522.x [DOI] [PubMed] [Google Scholar]
- Geschwind N., Galaburda A.M. Cerebral lateralization: biological mechanisms, associations, and pathology. I. A hypothesis and a program for research. Arch. Neurol. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Gesell A., Ames L.B. The development of handedness. J. Genet. Psychol. 1947;70:155–175. doi: 10.1080/08856559.1947.10533403. [DOI] [PubMed] [Google Scholar]
- Gilbert A.N., Wysocki C.J. Hand preference and age in the United States. Neuropsychologia. 1992;30:601–608. doi: 10.1016/0028-3932(92)90065-t. doi:10.1016/0028-3932(92)90065-T [DOI] [PubMed] [Google Scholar]
- Goldfield E.C., Michel G.F. The ontogeny of infant bimanual reaching during the first year. Infant Behav. Dev. 1986;9:81–89. doi:10.1016/0163-6383(86)90040-8 [Google Scholar]
- Goller F., Suthers R.A. Implications for lateralization of bird song from unilateral gating of bilateral motorpatterns. Nature. 1995;373:63–66. doi:10.1038/373063a0 [Google Scholar]
- Goodglass H., Quadfasel F.A. Language laterality in lefthanded aphasics. Brain. 1954;77:521–548. doi: 10.1093/brain/77.4.521. doi:10.1093/brain/77.4.521 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G. The ontogeny of social displays: form development, form fixation, and change in context. Adv. Study Behav. 1993;22:269–322. doi:10.1016/S0065-3454(08)60409-x [Google Scholar]
- Halpern D.F., Coren S. Do right-handers live longer? Nature. 1988;333:213. doi: 10.1038/333213b0. doi:10.1038/333213b0 [DOI] [PubMed] [Google Scholar]
- Harris L.J. Cultural influences on handedness: historical and contemporary theory and evidence. In: Coren S., editor. Left-handedness: behavioral implications and anomalies. Elsevier; Amsterdam, The Netherlands: 1990. pp. 195–258. [Google Scholar]
- Hepper P.G., Shahidullah S., White R. Handedness in the human fetus. Neuropsychologia. 1991;29:1107–1111. doi: 10.1016/0028-3932(91)90080-r. doi:10.1016/0028-3932(91)90080-R [DOI] [PubMed] [Google Scholar]
- Hepper P.G., Wells D.L., Lynch C. Prenatal thumb sucking is related to postnatal handedness. Neuropsychologia. 2005;43:313–315. doi: 10.1016/j.neuropsychologia.2004.08.009. doi:10.1016/j.neuropsychologia.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Hicks R.E., Kinsbourne M. Human handedness: partial cross-fostering study. Science. 1976;192:908–910. doi: 10.1126/science.1273577. doi:10.1126/science.1273577 [DOI] [PubMed] [Google Scholar]
- Hildreth G. The development and training of hand dominance. I. Characteristics of handedness. J. Genet. Psychol. 1949;75:197–220. doi: 10.1080/08856559.1949.10533517. [DOI] [PubMed] [Google Scholar]
- Hirnstein M., Hausmann M., Gunturkun O. The evolutionary origins of functional cerebral asymmetries in humans: does lateralization enhance parallel processing? Behav. Brain Res. 2008;187:297–303. doi: 10.1016/j.bbr.2007.09.023. doi:10.1016/j.bbr.2007.09.023 [DOI] [PubMed] [Google Scholar]
- Hopkins W.D. Heritability of hand preference in chimpanzees (Pan troglodytes): evidence from a partial interspecies cross-fostering study. J. Comp. Psychol. 1999;113:307–313. doi: 10.1037/0735-7036.113.3.307. doi:10.1037/0735-7036.113.3.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W.D., Dahl J.F., Pilcher D. Birth order and left-handedness revisited: some recent findings in chimpanzees (Pan troglodytes) and their implications for developmental and evolutionary models of human handedness. Neuropsychologia. 2000;38:1626–1633. doi: 10.1016/s0028-3932(00)00068-3. doi:10.1016/S0028-3932(00)00068-3 [DOI] [PubMed] [Google Scholar]
- Hopkins W.D., Dahl J.F., Pilcher D. Genetic influence on the expression of hand preferences in chimpanzees (Pan troglodytes): evidence in support of the right-shift theory and developmental instability. Psychol. Sci. 2001;12:299–303. doi: 10.1111/1467-9280.00355. doi:10.1111/1467-9280.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori M. Frequency-dependent natural-selection in the handedness of scale-eating cichlid fish. Science. 1993;260:216–219. doi: 10.1126/science.260.5105.216. doi:10.1126/science.260.5105.216 [DOI] [PubMed] [Google Scholar]
- Hori M., Ochi H., Kohda M. Inheritance pattern of lateral dimorphism in two cichlids (a scale eater, Perissodus microlepis, and an herbivore, Neolamprologus moorii) in Lake Tanganyika. Zool. Sci. 2007;24:486–492. doi: 10.2108/zsj.24.486. doi:10.2108/zsj.24.486 [DOI] [PubMed] [Google Scholar]
- Iwasaki S. Age and generation trends in handedness: an eastern perspective. In: Mandal M.K., Bulman-Fleming M.B., Tiwari G., editors. Side bias: a neuropsychological perspective. Kluwer; Dordrecht, The Netherlands: 2000. pp. 83–100. [Google Scholar]
- Iwasaki S., Kaiho T., Iseki K. Handedness trends across age-groups in a Japanese sample of 2316. Percept. Motor Skills. 1995;80:979–994. doi: 10.2466/pms.1995.80.3.979. [DOI] [PubMed] [Google Scholar]
- James W.H. Twinning, handedness and embryology. Percept. Motor Skills. 1983;56:721–722. doi: 10.2466/pms.1983.56.3.721. [DOI] [PubMed] [Google Scholar]
- Jansen A., Lohmann H., Scharfe S., Sehlmeyer C., Deppe M., Knecht S. The association between scalp hair-whorl direction, handedness and hemispheric language dominance: is there a common genetic basis of lateralization? Neuroimage. 2007;35:853–861. doi: 10.1016/j.neuroimage.2006.12.025. doi:10.1016/j.neuroimage.2006.12.025 [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Bolhuis J.J., Horn G. Interaction between acquired preferences and developing predispositions during imprinting. Anim. Behav. 1985;33:1000–1006. doi:10.1016/S0003-3472(85)80034-8 [Google Scholar]
- Jones G.V., Martin M. A note on Corballis (1997) and the genetics and evolution of handedness: developing a unified distributional model from the sex-chromosomes gene hypothesis. Psychol. Rev. 2000;107:213–218. doi: 10.1037/0033-295x.107.1.213. doi:10.1037/0033-295X.107.1.213 [DOI] [PubMed] [Google Scholar]
- Jordan H.E. The inheritance of left-handedness. Am. Breed. Mag. 1911;2:19–29. See also pp. 113–124. [Google Scholar]
- Kamping A., Van Delden W. A long-term study on interactions between the Adh and αGpdh allozyme polymorphisms and the chromosomal inversion In(2L)t in a seminatural population of D. melanogaster. J. Evol. Biol. 1999;12:809–821. doi:10.1046/j.1420-9101.1999.00083.x [Google Scholar]
- Klar A.J.S. A single locus, RGHT, specifies preference for hand utilization in humans. Cold Spring Harbor Symp. Quant. Biol. 1996;61:59–65. [PubMed] [Google Scholar]
- Konishi Y., Mikawa H., Suzuki J. Asymmetrical head-turning of preterm infants: some effects on lateral postural and functional lateralities. Dev. Med. Child Neurol. 1986;28:450–457. doi: 10.1111/j.1469-8749.1986.tb14282.x. [DOI] [PubMed] [Google Scholar]
- Konishi Y., Kuriyama M., Mikawa H., Suzuki J. Effect of body position on later postural and functional lateralities of preterm infants. Dev. Med. Child Neurol. 1987;29:751–757. doi: 10.1111/j.1469-8749.1987.tb08820.x. [DOI] [PubMed] [Google Scholar]
- Kuo Z.-Y., Shen T.C. Ontogeny of embryonic behavior in Aves. XI. Respiration in the chick embryo. J. Comp. Psychol. 1937;24:49–58. doi:10.1037/h0060969 [Google Scholar]
- Laland K.N., Kumm J., Vanhorn J.D., Feldman M.W. A gene-culture model of human handedness. Behav. Genet. 1995;25:433–445. doi: 10.1007/BF02253372. doi:10.1007/BF02253372 [DOI] [PubMed] [Google Scholar]
- Lansky L.M., Feinstein H., Peterson J.M. Demography of handedness in 2 samples of randomly selected adults (N=2083) Neuropsychologia. 1988;26:465–477. doi: 10.1016/0028-3932(88)90099-1. doi:10.1016/0028-3932(88)90099-1 [DOI] [PubMed] [Google Scholar]
- Laval S.H., et al. Evidence for linkage to psychosis and cerebral asymmetry (relative hand skill) on the X chromosome. Am. J. Med. Genet. B. 1998;81:420–427. doi: 10.1002/(sici)1096-8628(19980907)81:5<420::aid-ajmg11>3.0.co;2-e. doi:10.1002/(SICI)1096-8628(19980907)81:5<420::AID-AJMG11>3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- Leiber L., Axelrod S. Intra-familial learning is only a minor factor in manifest handedness. Neuropsychologia. 1981;19:273–288. doi: 10.1016/0028-3932(81)90111-1. doi:10.1016/0028-3932(81)90111-1 [DOI] [PubMed] [Google Scholar]
- Levin M. Twinning and embryonic left-right asymmetry. Laterality. 1999;4:197–208. doi: 10.1080/713754338. doi:10.1080/135765099396953 [DOI] [PubMed] [Google Scholar]
- Levy J. Psychobiological implications of bilateral asymmetry. In: Dimond S.J., Beaumont J.G., editors. Hemispheric function in the human brain. Paul Elek; London, UK: 1974. pp. 121–183. [Google Scholar]
- Levy J., Nagylaki T. Model for genetics of handedness. Genetics. 1972;72:117–128. doi: 10.1093/genetics/72.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaurens V., Raymond M., Faurie C. Why are some people left-handed? An evolutionary perspective. Phil. Trans. R. Soc. B. 2009;364:881–894. doi: 10.1098/rstb.2008.0235. doi:10.1098/rstb.2008.0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf E.V., Hopkins W.D. Wild chimpanzees show population-level handedness for tool use. Proc. Natl Acad. Sci. USA. 2005;102:12 634–12 638. doi: 10.1073/pnas.0505806102. doi:10.1073/pnas.0505806102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns M., Gunturkun O. Monocular deprivation alters the direction of functional and morphological asymmetries in the pigeon's (Columba livia) visual system. Behav. Neurosci. 1999;113(6):1257–1266. doi: 10.1037//0735-7044.113.6.1257. doi:10.1037/0735-7044.113.6.1257 [DOI] [PubMed] [Google Scholar]
- Mckeever W.F. A new family handedness sample with findings consistent with X-linked transmission. Br. J. Psychol. 2000;91:21–39. doi: 10.1348/000712600161655. doi:10.1348/000712600161655 [DOI] [PubMed] [Google Scholar]
- McManus I.C. Handedness, language dominance and aphasia: a genetic model. Psychol. Med. 1985a;8:1–40. [PubMed] [Google Scholar]
- McManus I.C. Right-hand and left-hand skill: failure of the right shift model. Br. J. Psychol. 1985b;76:1–16. doi: 10.1111/j.2044-8295.1985.tb01926.x. [DOI] [PubMed] [Google Scholar]
- McManus I.C. The inheritance of left-handedness. Ciba Found. Symp. 1991;162:251–281. doi: 10.1002/9780470514160.ch15. [DOI] [PubMed] [Google Scholar]
- McManus I.C. Are paw preference differences in hi and lo mice the result of specific genes or of heterosis and fluctuating asymmetry? Behav. Genet. 1992;22:435–451. doi: 10.1007/BF01066614. doi:10.1007/BF01066614 [DOI] [PubMed] [Google Scholar]
- McManus I.C. Handedness, cerebral lateralization, and the evolution of language. In: Corballis M.C., Lea S.E.G., editors. The descent of mind: psychological perspectives on hominid evolution. University Press; Oxford, UK: 1999. pp. 194–217. [Google Scholar]
- McManus I.C. Widenfeld and Nicolson; London, UK: 2002. Right hand, left hand. [Google Scholar]
- McManus I.C., Bryden M.P. The genetics of handedness, cerebral dominance and lateralization. In: Rapin I., Segalowitz S.J., editors. Handbook of neuropsychology. Elsevier; Amsterdam, The Netherlands: 1992. pp. 115–144. [Google Scholar]
- Medland S.E., Wright M.J., Geffen G.M., Hay D.A., Levy F., Martin N.G., Duffy D.L. Special twin environments, genetic influences and their effects on the handedness of twins and their siblings. Twin Res. 2003;6:119–130. doi: 10.1375/136905203321536245. doi:10.1375/136905203321536245 [DOI] [PubMed] [Google Scholar]
- Michel G.F. Right-handedness: a consequence of infant supine head-orientation preference? Science. 1981;212:685–687. doi: 10.1126/science.7221558. doi:10.1126/science.7221558 [DOI] [PubMed] [Google Scholar]
- Michel G.F., Goodwin R. Intrauterine birth position predicts newborn supine head position preferences. Infant Behav. Dev. 1979;2:29–38. doi:10.1016/S0163-6383(79)80005-3 [Google Scholar]
- Michel G.F., Tyler A.N., Ferre C., Sheu C.-F. The manifestation of infant hand-use preferences when reaching for object during the seven- to thirteen-month age period. Dev. Psychobiol. 2006;48:436–443. doi: 10.1002/dev.20161. doi:10.1002/dev.20161 [DOI] [PubMed] [Google Scholar]
- Morgan M.J., Corballis M.C. Biological basis of human laterality. 2. Mechanisms of inheritance. Behav. Brain Sci. 1978;1:270–277. [Google Scholar]
- Newman H.H. Studies of human twins. II. asymmetry reversal of mirror imaging in identical twins. Biol. Bull. 1928;55:298–315. doi:10.2307/1537082 [Google Scholar]
- Nottebohm F. Lateralization of bird song. Science. 1970;170:1333–1335. [Google Scholar]
- Nottebohm F. Neural lateralization of vocal control in a passerine bird. I. Song. J. Exp. Zool. 1971;177:229–261. doi: 10.1002/jez.1401770210. doi:10.1002/jez.1401770210 [DOI] [PubMed] [Google Scholar]
- Nottelmann F., Wohlslager A., Gunturkun O. Unihemispheric memory in pigeons: knowledge, the left hemisphere is reluctant to share. Behav. Brain Res. 2002;133:309–315. doi: 10.1016/s0166-4328(02)00011-6. doi:10.1016/S0166-4328(02)00011-6 [DOI] [PubMed] [Google Scholar]
- Oppenheim R.W. Prehatching and hatching behavior: comparative and physiological consideration. In: Gottlieb G., editor. Behavioral embryology. Academic Press; New York, NY: 1973. pp. 161–244. [Google Scholar]
- Orlebeke J.F., Knol D.L., Koopmans J.R., Boomsma D.I., Bleker O.P. Left-handedness in twins: genes or environment? Cortex. 1996;32:479–490. doi: 10.1016/s0010-9452(96)80005-0. [DOI] [PubMed] [Google Scholar]
- Pfannkuche K.A., Bouma A., Groothuis T.G.G. Does testosterone affect lateralization of brain and behaviour? A meta-analysis in humans and other animal species. Phil. Trans. R. Soc. B. 2009;364:929–942. doi: 10.1098/rstb.2008.0282. doi:10.1098/rstb.2008.0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porac C., Coren S. Springer; New York, NY: 1981. Lateral preferences and human behavior. [Google Scholar]
- Porac C., Coren S., Searleman A. Environmental-factors in hand preference formation: evidence from attempts to switch the preferred hand. Behav. Genet. 1986;16:251–261. doi: 10.1007/BF01070800. doi:10.1007/BF01070800 [DOI] [PubMed] [Google Scholar]
- Previc F.H. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol. Rev. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. doi:10.1037/0033-295X.98.3.299 [DOI] [PubMed] [Google Scholar]
- Provins K.A. Handedness and speech: a critical reappraisal of the role of genetic and environmental factors in the cerebral lateralization of function. Psychol. Rev. 1997;104:554–571. doi: 10.1037/0033-295x.104.3.554. doi:10.1037/0033-295X.104.3.554 [DOI] [PubMed] [Google Scholar]
- Rice T., Plomin R., DeFries J.C. Development of hand preference in the Colorado adoption project. Percept. Motor Skills. 1984;58:683–689. doi: 10.2466/pms.1984.58.3.683. [DOI] [PubMed] [Google Scholar]
- Riedstra, B. 2003 Development and social nature of feather pecking. PhD thesis, University of Groningen.
- Rife D.C. Handedness, with special reference to twins. Genetics. 1940;25:178–186. doi: 10.1093/genetics/25.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.J. Light experience and asymmetry of brain function in chickens. Nature. 1982;297:223–225. doi: 10.1038/297223a0. doi:10.1038/297223a0 [DOI] [PubMed] [Google Scholar]
- Rogers L.J. Light input and the reversal of functional lateralization in the chicken brain. Behav. Brain Res. 1990;38:211–221. doi: 10.1016/0166-4328(90)90176-f. doi:10.1016/0166-4328(90)90176-F [DOI] [PubMed] [Google Scholar]
- Rogers L.J. CAB International; Wallingford, UK: 1995. The development of brain and behaviour in the chicken. [Google Scholar]
- Rogers L. Behavioral, structural and neurochemical asymmetries in the avian brain: a model system for studying visual development and processing. Neurosci. Biobehav. Rev. 1996;20:487–503. doi: 10.1016/0149-7634(95)00024-0. doi:10.1016/0149-7634(95)00024-0 [DOI] [PubMed] [Google Scholar]
- Rogers L.J., Workman L. Footedness in birds. Anim. Behav. 1993;45:409–411. doi:10.1006/anbe.1993.1049 [Google Scholar]
- Ronnqvist L., Hopkins B. Motor asymmetries in the human newborn are state dependent, but independent of position in space. Exp. Brain Res. 2000;134:378–384. doi: 10.1007/s002210000467. doi:10.1007/s002210000467 [DOI] [PubMed] [Google Scholar]
- Ronnqvist L., Hopkins B., Van Emmerik R., de Groot L. Lateral biases in head turning and Moro response in the human newborn: are they both vestibular in origin? Dev. Psychobiol. 1998:339–349. doi: 10.1002/(sici)1098-2302(199812)33:4<339::aid-dev5>3.0.co;2-r. doi:10.1002/(SICI)1098-2302(199812)33:4<339::AID-DEV5>3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- Shafey T.M. Effect of lighted incubation on embryonic growth and hatchability performance of two strains of layer breeder eggs. Br. Poult. Sci. 2004;45:223–229. doi: 10.1080/00071660410001715821. doi:10.1080/00071660410001715821 [DOI] [PubMed] [Google Scholar]
- Shafey T.M., Al-Mohsen T.H. Embryonic growth, hatching time and hatchability performance of meat breeder eggs incubated under continuous green light. Asian–Austr. J. Anim. Sci. 2002;15:1702–1707. [Google Scholar]
- Sicotte N.L., Woods R.P., Mazziotta J.C. Handedness in twins: a meta-analysis. Laterality. 1999;4:265–286. doi: 10.1080/713754339. doi:10.1080/135765099396980 [DOI] [PubMed] [Google Scholar]
- Smart J.L., Jeffery C., Richards B. A retrospective study of the relationship between birth history and handedness at 6 years. Early Hum. Dev. 1980;4:79–88. doi: 10.1016/0378-3782(80)90011-0. doi:10.1016/0378-3782(80)90011-0 [DOI] [PubMed] [Google Scholar]
- Sommer I.E.C., Ramsey N.F., Bouma A., Kahn R.S. Cerebral mirror-imaging in a monozygotic twin. Lancet. 1999;354:1445–1446. doi: 10.1016/s0140-6736(99)04130-6. doi:10.1016/S0140-6736(99)04130-6 [DOI] [PubMed] [Google Scholar]
- Soper H.V., Satz P. Pathological left-handedness and ambiguous handedness: a new explanatory model. Neuropsychologia. 1984;22:511–515. doi: 10.1016/0028-3932(84)90046-0. doi:10.1016/0028-3932(84)90046-0 [DOI] [PubMed] [Google Scholar]
- Stocks P. A biometric investigation of twins and their brothers and sisters. Ann. Eugen. 1933;5:1–55. [Google Scholar]
- Subirana A. The relationship between handedness and cerebral dominance. J. Neurol. 1964;4:215–234. [PubMed] [Google Scholar]
- Templeton J.J., Gonzalez D.P. Reverse lateralization of visual discriminative abilities in the European starling. Anim. Behav. 2004;76:783–788. doi:10.1016/j.anbehav.2003.04.011 [Google Scholar]
- ten Cate C. Population lateralization in zebra finch courtship: a re-assessment. Anim. Behav. 1991;41:900–901. doi:10.1016/S0003-3472(05)80358-6 [Google Scholar]
- ten Cate C., Baauw A., Ballintijn M.R., Majoor B., Van der Horst I. Lateralization of orientation in sexually active zebra finches: eye use asymmetry or locomotor bias? Anim. Behav. 1990;39:992–995. doi:10.1016/S0003-3472(05)80968-6 [Google Scholar]
- Turkewitz G., Moreau T., Birch H.G. Head position and receptor organization in the human neonate. J. Exp. Child Psychol. 1966;4:169–177. doi: 10.1016/0022-0965(66)90017-8. doi:10.1016/0022-0965(66)90017-8 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Rogers L.J. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005;28:575–633. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Regolin L., Pagni P. Detour behaviour, imprinting and visual lateralization in the domestic chick. Cogn. Brain Res. 1999;7:307–320. doi: 10.1016/s0926-6410(98)00033-0. doi:10.1016/S0926-6410(98)00033-0 [DOI] [PubMed] [Google Scholar]
- Van Agtmael T., Forrest S.M., Del-Favero J., Van Broeckhoven C., Williamson R. Parametric and nonparametric genome scan analyses for human handedness. Eur. J. Hum. Genet. 2003;11:779–783. doi: 10.1038/sj.ejhg.5201048. doi:10.1038/sj.ejhg.5201048 [DOI] [PubMed] [Google Scholar]
- Yeo R.A., Gangestad S.W. Developmental origins of variation in human hand preference. Genetica. 1993;89:281–296. doi:10.1007/BF02424521 [Google Scholar]