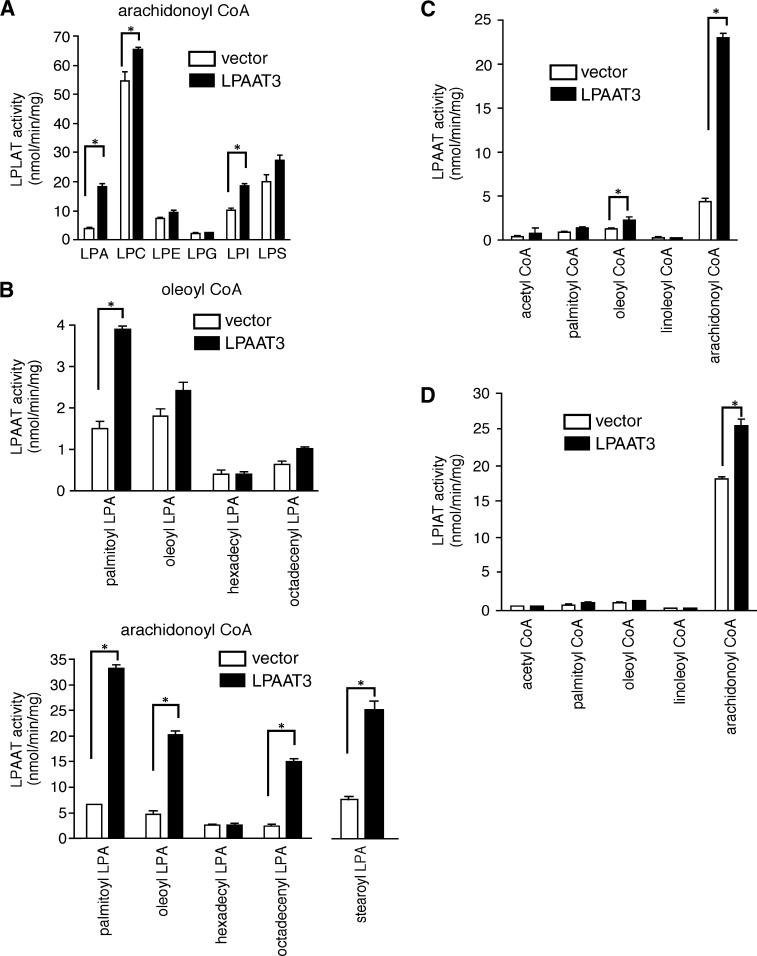
Fig. 4.
Substrate selectivity of mLPAAT3. A: Lysophospholipid preferences of mLPAAT3 were determined. Acyltransferase activity was examined using 2 μg protein (100,000 g pellet), 25 μM [1-14C]arachidonoyl-CoA (33,000 dpm), and 50 μM lysophospholipids. Data are shown as mean + SD of triplicate measurements. Statistical significance was analyzed using ANOVA with Tukey post hoc pairwise comparisons. *P < 0.05. B: The preference of mLPAAT3 for various LPA acceptors was examined using oleoyl-CoA or arachidonoyl-CoA as a donor. Acyltransferase activity was examined using 2 μg protein, 25 μM [1-14C]oleoyl-CoA (33,000 dpm), and 50 μM lysophospholipids. Data are shown as mean + SD of triplicate measurements. Statistical significance was analyzed using ANOVA with Tukey post hoc pairwise comparisons. Only for stearoyl-LPA group, t-test was used for analysis. *P < 0.05. C: The acyl-CoA selectivity of mLPAAT3 was examined using palmitoyl-LPA as an acceptor. Acyltransferase activity was examined using 2 μg protein, 25 μM acyl-CoAs (33,000 dpm), and 50 μM palmitoyl-LPA, with the exception that 100 μM acetyl-CoA (111,000 dpm, 185MBq / mmol) was used. Data are shown as mean + SD of triplicate measurements. Statistical significance was analyzed using ANOVA with Tukey post hoc pairwise comparisons. *P < 0.05. D: The acyl-CoA selectivity of mLPAAT3 was examined for LPIAT activity. Acyltransferase activity was examined using 2 μg protein, 25 μM acyl-CoAs (33,000 dpm), and 50 μM LPI with exception of acetyl-CoA. The concentration of acetyl-CoA used was 100 μM (111,000 dpm, 185MBq/mmol). Data are shown as mean + SD of triplicate measurements. Statistical significance was analyzed using ANOVA with Tukey post hoc pairwise comparisons. *P < 0.05. In A–D, results are representative of two independent experiments.