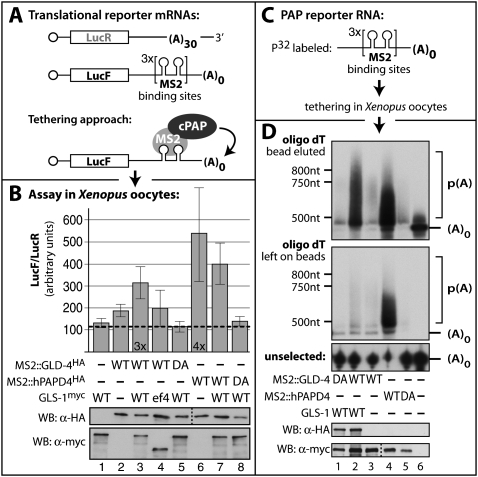
Figure 4.
GLD-4 is a PAP and requires GLS-1 for biochemical activity. (A) Translational simulation tested by a tethering assay in Xenopus oocytes. Schematic diagram of mRNAs encoding LucF and LucR. The LucR reporter mRNA contains a poly(A) tail of 30 adenosines (A). The LucF reporter contains MS2 RNA-binding sites in its 3′UTR and no poly(A) tail. MS2-fused PAPs are specifically recruited to the LucF mRNA reporter. (B) Measurements of relative luciferase activities from oocyte extracts. The expression levels of all test proteins are detected with Tag-specific antibodies. The translation of the LucF reporters is stimulated by wild-type (WT) hPAPD4 alone and by GLD-4 mostly the presence of wild-type GLS-1. Catalytic inactive mutants (DA) of both PAPs have no effect on translational enhancement. Data represented as ±SEM. (C) Tethering assay to asses the length of the poly(A) tail with a smaller MS2 RNA substrate. (D) GLD-4 and GLS-1 are required for efficient poly(A) tail elongation. Total RNA was extracted and poly(A) RNA selected on oligo dT beads. A water eluate is given on the top and the remaining strongly bead-bound RNAs were denatured and heat-eluted (middle). RNA polyadenylation is visible as an upward smear in polyacylamide gels. (Bottom) The unselected RNA in the extract supernatant is given as an integrity control.