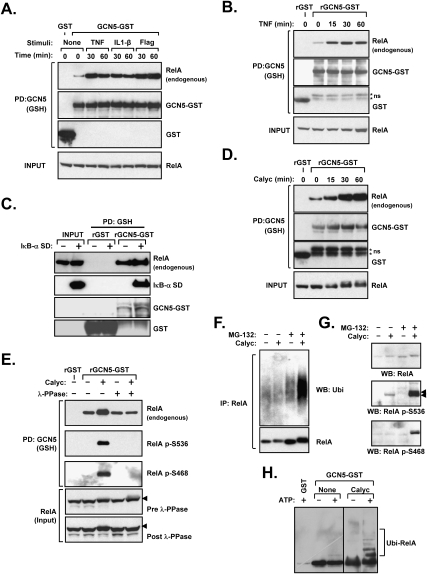
Figure 4.
RelA phosphorylation enhances GCN5-RelA interactions. (A) Various IKK-activating stimuli promote GCN5–RelA binding. HEK293 cells were transfected with GCN5–GST or GST and subsequently treated with TNF, IL-1β, or Flagellin prior to lysis and GSH precipitation. The presence of coprecipitated endogenous RelA was determined by immunoblotting. (B) TNF treatment prior to lysis promotes RelA–GCN5 binding in vitro. HEK293 cells were treated with TNF or Calyculin A, and the obtained lysates were mixed with bacterially made rGST or rGCN5–GST, followed by precipitation. The presence of endogenous RelA in the recovered material was determined by immunoblotting. (C) IκB does not impair the GCN5–RelA interaction. HEK293 cells were transfected with IκB-α superdominant (SD), and the ability of recombinant GCN5 to precipitate endogenous RelA post-lysis was examined as in B. (D) The phosphatase inhibitor Calyculin A promotes RelA–GCN5 binding in vitro. HEK293 cells were treated with Calyculin A and the obtained lysates were mixed with rGST or rGCN5–GST, followed by precipitation. The presence of endogenous RelA in the recovered material was determined by immunoblotting. (E) Dephosphorylation of RelA abrogates its induced binding to GCN5. HEK293 cells were treated with Calyculin A and subsequently lysed in a buffer without phosphatase inhibitors. The lysate was incubated with λ-protein phosphatase (λ-PPase) as indicated, and in vitro binding of RelA to recombinant GCN5 was performed as in D. The recovered material was immunoblotted to detect the coprecipitation of endogenous RelA and phosphorylated RelA. (F) Phosphorylation promotes RelA ubiquitination. Wild-type MEFs were treated with MG-132 (30 min) and Calyculin A as indicated. RelA was subsequently immunoprecipitated from cell lysates and the presence of ubiquitinated RelA was determined by immunoblotting for ubiquitin. (G) Phosphorylated RelA is labile and is stabilized by proteasomal blockade. Wild-type MEFs were treated with Calyculin A and MG-132 as indicated. Phosphorylated RelA levels were determined by Western blot using two phospho-specific antibodies. (H) GCN5-associated ligase activity ubiquitinates RelA in vitro. HEK293 cells transfected with GCN5–GST or GST were treated without or with Calyculin A as shown. Material precipitated by GSH beads was applied to an in vitro ubquitination reaction as in Figure 3A. Coprecipitated RelA and its ubiquitination were shown by immunoblotting.