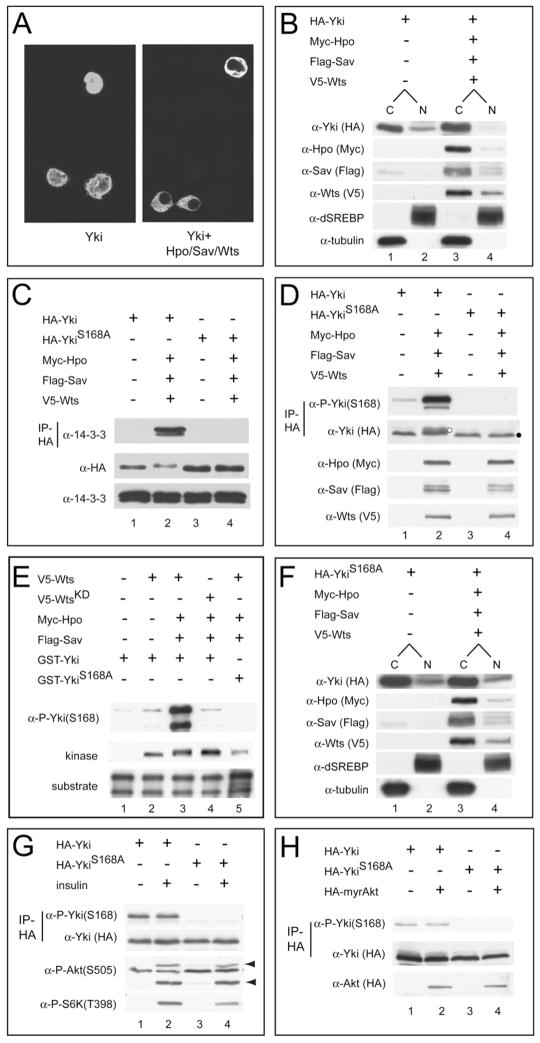
Figure 1. Hippo Signaling Phosphorylates Yki S168 and Promotes Its Cytoplasmic Localization.
(A) S2 cells expressing HA-Yki (left) or HA-Yki plus Hpo, Sav, and Wts plasmids (right) were stained with α-HA antibody. Nuclear HA-Yki signal is detected in 100% of cells expressing HA-Yki alone, but in only 9% of cells coexpressing Hpo-Sav-Wts.
(B) Cells were transfected as in (A) and analyzed by subcellular fractionation. Note the decrease of nuclear Yki under active Hippo signaling (compare lanes 2 and 4). dSREBP (nuclear) and tubulin (cytosolic) were used as quality control for fractionation. C, cytoplasmic; N, nuclear.
(C) Cells were transfected as in (A), and α-HA immunoprecipitates were probed with α-14-3-3 (top gel). Cell lysates were also probed with the indicated antibodies (middle and bottom gels). Yki, but not YkiS168A, immunoprecipitated 14-3-3 under active Hippo signaling.
(D) Cells were transfected as in (A), and α-HA immunoprecipitates were probed with α-P-Yki(S168) and α-HA antibodies (top two gels). Cell lysates were also probed with the indicated antibodies (bottom three gels). Yki, but not YkiS168A, showed S168 phosphorylation, which was further increased under active Hippo signaling. Also note that Yki, but not YkiS168A, showed mobility shift under active Hippo signaling (compare lanes 2 and 4; indicated by white and black dots, respectively).
(E) Wts phosphorylates Yki at S168 in vitro. V5-tagged Wts (or kinase-dead WtsKD) was expressed alone or together with Hpo-Sav in S2 cells, immunoprecipitated, and incubated with GST-Yki (or GST-YkiS168A), and the reaction products were probed with α-P-Yki(S168). The input kinase and substrate are also shown (bottom two gels). Note that our GST-Yki preparation contains two Yki-related bands (Huang et al., 2005). Strong S168 phosphorylation was detected when Wts (lane 3), but not WtsKD (lane 4), was coexpressed with Hpo-Sav. No S168 phosphorylation was detected using GST-YkiS168A as a substrate (lane 5).
(F) Subcellular fractionation of YkiS168A mutant. The relative proportion of cytoplasmic and nuclear YkiS168A was not changed under active Hippo signaling.
(G) S2 cells expressing HA-Yki or HA-YkiS168A were treated with or without insulin. α-HA immunoprecipitates were probed with the indicated antibodies (top two gels). Cell lysates were also probed with the P-S6K or P-Akt antibodies (bottom two gels). Arrowheads mark the phospho-Akt signals. Insulin stimulates the phosphorylation of Akt and S6K, but not that of Yki (compare lanes 1 and 2).
(H) S2 cells expressing HA-Yki or HA-YkiS168A plus a constitutively active Akt mutant (myr-Akt) were analyzed as in (G). Similar P-Yki(S168) levels were seen in the presence or absence of myr-Akt (compare lanes 1 and 2).