Abstract
Normally, meiotic crossovers in conjunction with sister-chromatid cohesion establish a physical connection between homologs that is required for their accurate segregation during the first meiotic division. However, in some organisms an alternative mechanism ensures the proper segregation of bivalents that fail to recombine. In Drosophila oocytes, accurate segregation of achiasmate homologs depends on pairing that is mediated by their centromere-proximal heterochromatin. Our previous work uncovered an unexpected link between sister-chromatid cohesion and the fidelity of achiasmate segregation when Drosophila oocytes are experimentally aged. Here we show that a weak mutation in the meiotic cohesion protein ORD coupled with a reduction in centromere-proximal heterochromatin causes achiasmate chromosomes to missegregate with increased frequency when oocytes undergo aging. If ORD activity is more severely disrupted, achiasmate chromosomes with the normal amount of pericentric heterochromatin exhibit increased nondisjunction when oocytes age. Significantly, even in the absence of aging, a weak ord allele reduces heterochromatin-mediated pairing of achiasmate chromosomes. Our data suggest that sister-chromatid cohesion proteins not only maintain the association of chiasmate homologs but also play a role in promoting the physical association of achiasmate homologs in Drosophila oocytes. In addition, our data support the model that deterioration of meiotic cohesion during the aging process compromises the segregation of achiasmate as well as chiasmate bivalents.
IN both mitotic and meiotic cells, cohesion between sister chromatids is essential for accurate chromosome segregation (Lee and Orr-Weaver 2001). Cohesion depends on the evolutionarily conserved cohesin complex that consists of two structural maintenance of chromosomes (SMC) and two non-SMC proteins (Uhlmann 2001). In addition to holding sisters together, meiotic cohesion is also required to keep recombinant homologs physically associated prior to their segregation (Buonomo et al. 2000; Bickel et al. 2002; Hodges et al. 2005). Chiasma maintenance relies on cohesion between the arms of sister chromatids (see Figure 1) and separase-mediated release of arm cohesion is necessary for homolog disjunction at anaphase I (Buonomo et al. 2000; Pasierbek et al. 2001; Siomos et al. 2001; Kudo et al. 2006).
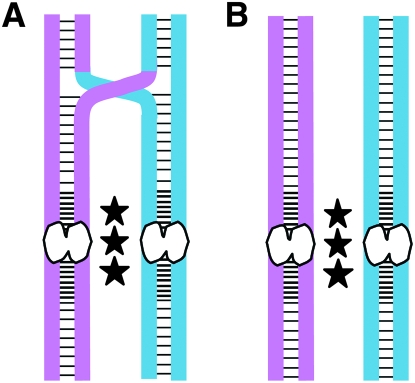
Figure 1.—
Mechanisms that ensure the association of homologous chromosomes in Drosophila oocytes. Pink and blue are used to differentiate the homologous chromosomes (each composed of two sister chromatids). Sister-chromatid cohesion proteins are depicted as black horizontal lines. In Drosophila oocytes, cohesion proteins (SMC1, SMC3, and ORD) are highly enriched near the centromeres (white bilobed structures). Black stars represent heterochromatin-mediated pairing of homologous chromosomes that is required for accurate segregation of achiasmate bivalents. (A) Cohesion along the arms of sister chromatids provides an evolutionarily conserved mechanism to maintain the association of recombinant homologs until anaphase I. (B) In Drosophila oocytes, bivalents that lack a crossover rely on the achiasmate pathway to keep homologous chromosomes associated and thereby ensure their proper segregation. (Note that heterochromatin-mediated pairing of chiasmate homologs also occurs, but is not required for their accurate disjunction unless chiasmata are not maintained.)
Although proper segregation of homologous chromosomes during meiosis I generally requires formation and maintenance of chiasmata, several organisms harbor an alternate mechanism that ensures faithful disjunction of bivalents that fail to recombine (Wolf 1994). Accurate segregation of achiasmate bivalents has been reported in Saccharomyces cerevisiae, Schizosaccharomyces pombe, Caenorhabditis elegans, and Drosophila melanogaster (Dawson et al. 1986; Guacci and Kaback 1991; Hawley et al. 1993; Ross et al. 1996; Molnar et al. 2001; Meneely et al. 2002; Davis and Smith 2005; Doll et al. 2005). In budding yeast, centromere pairing of achiasmate chromosomes facilitates their accurate disjunction in meiosis I (MI) (Dawson et al. 1986; Guacci and Kaback 1991; Ross et al. 1996; Kemp et al. 2004). In addition, the checkpoint protein Mad3 (orthologous to BubR1 in metazoans) also plays a role in directing the proper segregation of the nonrecombinant bivalents in S. cerevisiae, although the exact mechanism by which Mad3 facilitates achiasmate segregation is not clear (Cheslock et al. 2005). In fission yeast, the microtubule motor dynein promotes pairing between homologous chromosomes during prophase I, and mutations in dlc1 (dynein light chain) cause missegregation of achiasmate bivalents (Molnar et al. 2001; Davis and Smith 2005). In addition, achiasmate homologs in S. pombe also exhibit centromere pairing (Ding et al. 2004). Although accurate disjunction of achiasmate autosomes has been observed during C. elegans spermatogenesis, the molecular mechanisms underlying this pathway have not been explored (Meneely et al. 2002).
The molecules and mechanisms governing achiasmate chromosome segregation have been most thoroughly investigated in fruit flies. In Drosophila oocytes, accurate segregation of achiasmate bivalents depends upon homology-based association of homologs within their centromere-proximal heterochromatin (see Figure 1). Minichromosomes carrying duplications of pericentric heterochromatin compete for pairing sites on bivalents and cause MI nondisjunction (NDJ) if the bivalents are achiasmate (Hawley et al. 1993). Furthermore, there is a linear relationship between the amount of pericentric heterochromatin and the fidelity of achiasmate segregation (Karpen et al. 1996). Cytological analyses of Drosophila oocytes indicate that the centromere-proximal heterochromatin of homologous chromosomes remains associated from pachytene until anaphase I (Dernburg et al. 1996; Gilliland et al. 2009; Hughes et al. 2009). However, whether or how specific heterochromatin proteins promote this association has not been investigated.
In Drosophila, orientation disruptor (ORD) is essential for sister chromatid cohesion during meiosis in both sexes (Mason 1976; Miyazaki and Orr-Weaver 1992; Bickel et al. 1996; Balicky et al. 2002; Webber et al. 2004). In the absence of ORD activity, chromosomes segregate randomly during both meiosis I and meiosis II, consistent with complete absence of both arm and centromere cohesion (Miyazaki and Orr-Weaver 1992; Bickel et al. 1996, 1997). ORD function is also required for normal levels of homologous exchange in Drosophila oocytes (Mason 1976; Miyazaki and Orr-Weaver 1992; Bickel et al. 1997; Webber et al. 2004). Additional studies have shown that the recombination defect in ord mutant oocytes arises because sister-chromatid exchange is elevated, resulting in a lower number of crossovers between homologous chromosomes (Webber et al. 2004). In addition, consistent with its role in arm cohesion, ORD is also required for chiasma maintenance until anaphase I (Bickel et al. 2002). Although some crossovers occur between homologous chromosomes in ord mutant oocytes, these bivalents still missegregate during meiosis I because chiasmata are not maintained (Mason 1976; Miyazaki and Orr-Weaver 1992; Bickel et al. 1997).
In Drosophila oocytes, ORD colocalizes extensively with cohesin subunits along the chromosome arms and like cohesin, ORD is enriched at the centric/pericentric regions of meiotic chromosomes (Webber et al. 2004; Khetani and Bickel 2007). In support of its essential role in meiotic cohesion, ORD activity is required for localization of SMC1 and SMC3 at the centromeres of meiotic chromosomes and their enrichment at the pericentric heterochromatin (Khetani and Bickel 2007). In addition, previous findings have revealed an unexpected link between ORD and achiasmate chromosome segregation during meiosis I in Drosophila oocytes (Jeffreys et al. 2003). When Drosophila oocytes are subjected to aging and ORD function is compromised by a weak mutation, meiotic NDJ of achiasmate bivalents is significantly higher in “aged oocytes” than in “nonaged oocytes” (Jeffreys et al. 2003). Although it is well established that cohesion proteins along the chromatid arms are required to hold chiasmate homologs together (see Figure 1), the finding that a sister-chromatid cohesion protein is required for accurate segregation of achiasmate homologs is novel. One possibility consistent with these data is that in addition to holding sister centromeres together, cohesion proteins enriched at the pericentric heterochromatin also play a role in holding achiasmate homologs together (see Figure 1).
In this article, we further explore the mechanism by which a sister-chromatid cohesion protein contributes to the accurate segregation of achiasmate bivalents. We show that when ORD function is compromised by a weak mutation and centromere-proximal heterochromatin also is reduced, achiasmate chromosomes become more susceptible to NDJ when oocytes undergo aging. Furthermore, in the presence of a stronger ord allele, achiasmate chromosomes with the normal amount of pericentric heterochromatin exhibit increased nondisjunction when oocytes age. Significantly, even in the absence of aging, a weak ORD allele disrupts heterochromatin-mediated pairing of achiasmate chromosomes. Our results indicate that the cohesion protein ORD promotes the physical association of achiasmate homologs. In addition, our data provide further support for the model that meiotic cohesion declines with age and argue that as cohesion proteins deteriorate with age, so does the fidelity of achiasmate segregation.
MATERIALS AND METHODS
Drosophila stocks and genetics:
Flies were reared at 25° on standard cornmeal molasses media. The ord4, ord8, and ord10 alleles used in this study have been characterized previously (Miyazaki and Orr-Weaver 1994; Bickel et al. 1996, 1997). The ord4 (A424V) and ord8 (H366Y) mutations reside in the last quarter of the ORD open reading frame and result in low and moderate NDJ levels (respectively) in mutant oocytes. ord10 is a nonsense allele (L24STOP) that behaves as a genetic null. Descriptions of the other genetic markers and chromosomes used can be found at http://www.flybase.org.
Recombination analysis:
To assay X chromosome crossover frequency and distribution in ord oocytes, 7–10 females were crossed to 5 yw males per vial. Crossover frequency and distribution were measured between the “y sc cv v f car” and “y” X homologs in experimental females by assaying sc, cv, v, f, car markers in their male progeny. The recombination data were used to estimate tetrad exchange ranks (Weinstein 1936).
Aging regimen and generation of 24-hr broods:
We have previously described an aging regimen that causes Drosophila oocytes to halt in development within the ovariole and “age” (Jeffreys et al. 2003). This experimentally induced aging mimics the normal aging process that human oocytes undergo during the lifetime of the female. In this study, we modified the aging regimen described by Jeffreys et al. (2003) such that the glucose agar media was prepared without the addition of fungal inhibitors (methyl paraben and ethyl acetate). We have found that omission of fungal inhibitors in the media during the aging regimen reduces the absolute level of NDJ in our assay; therefore, NDJ values in this study are lower than previously reported (Jeffreys et al. 2003). The glucose agar media contained 2% agar (Fisher) and 5% dextrose (Fisher) and was prepared with milli-Q grade water. Yeast paste was prepared by dissolving 30 g of active dry yeast (Red Star) in 50 ml milli-Q grade water.
A schematic of the aging regimen and the NDJ assay are shown in Figure 2, A and B. Approximately 200 virgin females of the desired genotype were collected within an 8-hr period and the females were fed yeast overnight in vials with cornmeal molasses media to promote yolk deposition and maturation of oocytes. This ensured that the ovaries contained a complete complement of oocytes at the different stages. The following day, females were split into two groups and placed in separate plexiglass laying bottles containing a glucose agar plate with a smear of yeast paste. Control and experimental flies were held in the laying bottles for 4 days with fresh yeast paste/glucose-agar plates supplied each day. The control group of females was supplied with an equal number of male flies and laid their eggs continuously. Their oocytes were “nonaged.” The experimental group of females was deprived of males. Because oviposition is suppressed in these females, the majority of the oocytes (stages 1–8) halt in developmental progression and age within the female abdomen (Subramanian and Bickel 2008). Stages 9–13 continue to progress through oogenesis even when oviposition is suppressed, but then arrest at stage 14, resulting in a large excess of mature oocytes that also age for the remainder of the regimen (King 1957; Subramanian and Bickel 2008). Figure 2C depicts the relative distribution of different oocyte stages in nonaged vs. aged ovarioles.
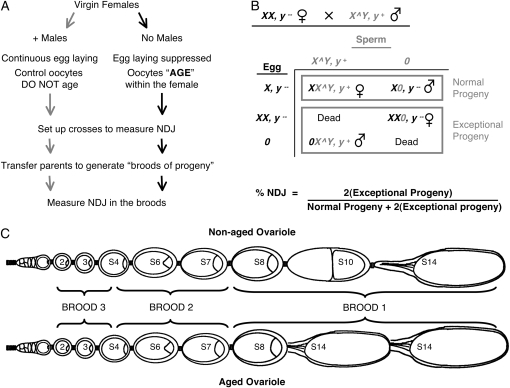
Figure 2.—
Scheme to age oocytes and measure NDJ (A) To age oocytes in Drosophila, egg laying is suppressed in virgin females. Virgin females of the same age and genotype are split into two groups. The control group of females is allowed to mate and their oocytes do not age. In contrast, the experimental group of females is not allowed to mate; in these females egg laying is suppressed and the oocytes age within the female. After females are subjected to the aging regimen for 4 days, crosses are set up to measure NDJ (see B). The parents are transferred at 24-hr intervals to generate broods of progeny that are assayed for NDJ during maternal meiosis. (B) To measure X chromosome NDJ during female meiosis, we used the recessive body color mutation, yellow (y). Experimental females that are y− are crossed to males whose X and Y chromosomes are physically linked (X^Y). The sperm from these males will have either an X^Y chromosome marked with y+ or no sex chromosomes (designated “0”). If meiotic chromosome segregation is normal, the oocyte will contain one X chromosome. Missegregation of X chromosomes may result in an oocyte with two X chromosomes (diplo X, y−) or no X chromosomes (Nullo, “0”). The exceptional progeny that arise due to meiotic NDJ can be distinguished from the progeny that arise from normal meiosis on the basis of their sex and body color. In this NDJ test, only half of the exceptional progeny survive; therefore, the number of exceptional progeny is doubled and the total number of progeny is similarly adjusted to calculate % NDJ. (C) The Drosophila ovary is composed of 15–30 ovarioles each containing a linear array of oocytes at progressive stages of development (stages 1–14). Oocytes at different stages can be distinguished on the basis of their size and morphology. Meiosis is initiated at the anterior end of the ovariole (left) and the oocyte moves posteriorly as it develops. Not all oocyte stages are present in the ovariole at any given time. The schematic illustrates which oocyte stages in aged and nonaged ovarioles give rise to each 24-hr brood of progeny after completion of the aging regimen. When egg laying is suppressed (aged ovariole), stage 14 oocytes accumulate at the expense of stages 9–13 (Subramanian and Bickel 2008). The schematic is adapted from Robinson et al. (1994).
At the end of the 4-day aging regimen, the experimental females (with aged oocytes) and the control females (with nonaged oocytes) were crossed to X^Y, v f B males to measure meiotic nondisjunction in the oocytes (see Figure 2B). To generate 24-hr broods, 10 female flies were mated with 5 X^Y, v f B males (per vial). The parents were transferred to new vials every 24-hr and three broods of progeny were analyzed for NDJ.
Nondisjunction assay:
Because Drosophila can tolerate certain sex chromosome aneuploidies, segregation errors during meiosis can be monitored in the viable progeny by using differentially marked sex chromosomes (Figure 2B). To compensate for the fact that only half of the exceptional progeny survive, total NDJ was calculated as [2 × exceptional progeny/(2 × exceptional progeny + normal progeny)] × 100.
For some NDJ tests, we performed an additional cross to genotype the X chromosomes in the diplo-X progeny (exceptional progeny that received two X chromosomes from the mother) as previously described (Subramanian and Bickel 2008). This allowed us to determine the recombinational history of missegregating chromosomes and/or determine whether missegregation events were reductional or equational.
Generation of probes for in situ and FISH analysis in whole mount ovaries:
A portion of the centromere proximal heterochromatin on the X chromosome consists of a 359-bp repeat sequence that spans 11 Mb. PCR amplification of the 359-bp repeat from wild-type Drosophila genomic DNA was performed using primers previously described (Dernburg et al. 1996) and the PCR product was digested overnight with Tsp509I restriction enzyme at 65° in a thermocycler. The 100-bp fragment was labeled with dUTP-fluorogreen using terminal transferase to generate the probe as described in Bickel et al. (2002).
FM7a/y w and FM7a/y;ord4 bw/cn ord10 bw oocytes were hybridized with the 359-bp probe. Ovaries from 15 females per sample were hand-dissected in 1× modified Robb's buffer (Theurkauf and Hawley 1992). The ovaries were fixed for 4 min in 4% formaldehyde/100 mm Na cacodylate (pH 7.2)/100 mm sucrose/40 mm K acetate/10 mm Na acetate/10 mm EGTA, prewarmed at 37°. In situ hybridization of the probes (mentioned above) was performed using the published protocol from Dernburg et al. (1996).
Microscopy and image analysis:
Epifluorescence imaging was carried out using a Zeiss Axioimager M1 microscope equipped with a Hamamatsu ORCA-ER camera. Images of ovarioles were captured using a 100× Plan-Apochromat (NA 1.4) objective and Openlab software (Improvision, version 4.0.4), whereas a 63× Plan-Apochromat (NA 1.4) was used for scoring the samples. Image stacks (0.1 μm step size) were deconvolved and cropped using Volocity software from Improvision. Openlab software was used to pseudocolor the images (projection of z-stacks).
Data analysis and statistical significance:
The frequency of missegregation is plotted as % NDJ. The error bars represent 95% confidence limits that were calculated using the extended Wald method (Agresti and Coull 1998). All statistical analyses were performed using a 2 × 2 χ2 contingency test. To compare aged vs. nonaged NDJ values, nonadjusted data were used for the statistical tests. For all tests, a two-tailed P-value of <0.05 was considered statistically significant (rejection of the null hypothesis that the two groups are the same).
RESULTS
Different ord alleles disrupt achiasmate segregation in aged oocytes:
Cohesion proteins not only provide a mechanism to hold sister chromatids together but also are required to maintain the association of chiasmate homologs during meiosis (Buonomo et al. 2000; Bickel et al. 2002; Hodges et al. 2005). Moreover, our recent work indicates that when Drosophila oocytes undergo aging, deterioration of meiotic cohesion causes loss of chiasmata and missegregation of recombinant homologs during meiosis I (Subramanian and Bickel 2008).
The aging regimen that we have developed for Drosophila oocytes mimics the normal aging process of human oocytes as women grow older (Jeffreys et al. 2003; Subramanian and Bickel 2008). Briefly, when virgin females are deprived of males, the developmental progression of their oocytes halts and the oocytes age within the females (see Figure 2). Although the age of the female fly does not dictate the age of her oocytes, for simplicity we will refer to a significant increase in NDJ when the oocytes are experimentally aged as “age-dependent NDJ.” Because meiotic cohesion weakens as oocytes age, we have been able to detect meiotic segregation defects that are not apparent under normal conditions (no aging) (Jeffreys et al. 2003; Subramanian and Bickel 2008). Notably, this approach led to the surprising finding that when oocytes undergo aging, mutation of the cohesion protein ORD disrupts the segregation of obligate achiasmate homologs (Jeffreys et al. 2003).
To further investigate the role of ORD in the segregation of achiasmate chromosomes, we reevaluated and extended our analysis of meiotic NDJ in females with ord mutations that have a weak or moderate effect on meiotic cohesion. Under normal conditions (no aging regimen) the weakest ord allele, ord4, results in 2.2% sex chromosome NDJ in oocytes and 0.6% in spermatocytes when placed in trans to an ordnull allele (Miyazaki and Orr-Weaver 1992; Bickel et al. 1996). The slightly stronger ord8 allele when placed over a null allele results in 10.9% sex chromosome NDJ in oocytes and 4.0% in spermatocytes (Bickel et al. 1997). Because in wild-type fruit flies, crossovers do not occur during male meiosis and arm cohesion is released prior to spindle assembly (Vazquez et al. 2002), the NDJ observed in ord mutant spermatocytes can be attributed to defects in centromeric cohesion. Therefore, the meiotic NDJ in males indicate that the centromeric function of ORD is more severely compromised by the ord8 mutation than by the ord4 mutation. For the ordnull allele in the experiments described below, we utilized ord10, which truncates the ORD open reading frame early in the coding region and behaves as a null in genetic tests (Bickel et al. 1997). Because of a slight modification of our aging regimen (see materials and methods), we repeated our previous analysis of age-dependent NDJ in X/X; ord4/ord10 and FM7a/X;ord4/ord10 oocytes (Jeffreys et al. 2003) for this study. These two genotypes provide the foundation for additional experiments described below.
When we subjected ord4/ord10 female flies with normal X chromosomes to our 4-day aging regimen (see materials and methods), sex chromosome NDJ was not significantly greater in aged oocytes than in nonaged oocytes (supplemental Figure S1A and Table S1). These data confirm our previous results that the weak ord4 mutation does not cause normal X chromosomes to become more vulnerable to missegregation during the aging process.
To specifically examine the role of ORD activity in the segregation of achiasmate chromosomes, we subjected FM7a/X;ord4/ord10 females to the aging regimen. FM7a is an X chromosome balancer that contains multiple inversions along its length (compare Figure 3, A and B). Apart from inversions in the euchromatin that suppress recombination, the pericentric heterochromatin of FM7a is rearranged such that a large portion is displaced to the distal end of the chromosome (Figure 3B). In a female fly with a normal X chromosome and an FM7a balancer, meiotic crossovers on the X chromosome are suppressed, and the X bivalents (FM7a/X) depend entirely on the achiasmate pathway for segregation in meiosis I. Following the aging regimen, FM7a/X;ord4/ord10 aged oocytes exhibited a significant increase in NDJ that lasted for 48 hr (two 24-hr broods) (supplemental Figure S1B and Table S2). Our analysis of diplo-X females (exceptional progeny that received two X chromosomes from the mother) indicated that reductional NDJ events predominated in both aged and nonaged oocytes (FM7a/X diplos: 107/107 for aged, 43/44 for nonaged). Therefore, although the ord4 mutation weakens sister-chromatid cohesion, it rarely results in complete separation of sister chromatids even when oocytes undergo aging (as evidenced by the low number of equational exceptions). Together, these data confirm our previous findings that when ord4/ord10 oocytes undergo aging, obligate achiasmate chromosomes become more susceptible to missegregation during the first meiotic division.
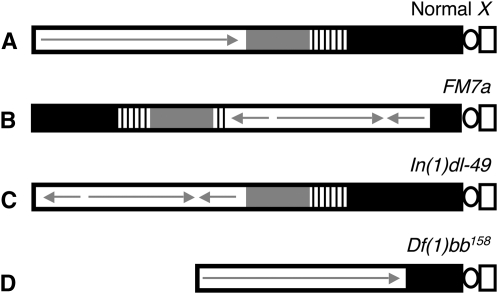
Figure 3.—
Diagram illustrating the different X chromosomes used in NDJ tests. (A) A normal X chromosome is shown with the centromeric constriction on the right. Euchromatin is depicted as a white rectangle, with a continuous arrow indicating a lack of rearrangements. The shaded and striped regions represent heterochromatin with the 11-Mb satellite DNA (359-bp repeat) (solid) and rDNA (stripes). (B) The FM7a balancer chromosome contains multiple inversions within the euchromatin (represented with arrows) as well as a rearrangement that places a large region of centromere-proximal heterochromatin at the distal end of the chromosome. (C) The In(1)dl-49 chromosome contains an inversion in the euchromatin that reduces recombination to ∼20% of wild type. However, centromere-proximal heterochromatin is unaffected. (D) The Df(1)bb158 contains a deletion that removes ∼80% of the centromere-proximal heterochromatin but the euchromatic region of the chromosome is normal.
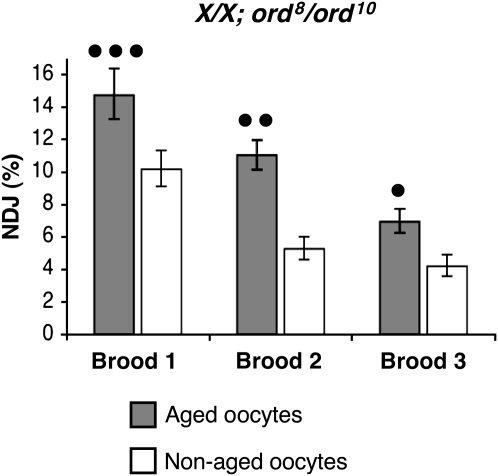
We next analyzed the effect of aging on the segregation of normal X/X bivalents in ord8/ord10 oocytes. For this genotype, we observed significant age-dependent NDJ even in the absence of an FM7a balancer chromosome (Figure 4, supplemental Table S3). In addition, normal X chromosomes in ord8/ord10 flies exhibited significantly higher NDJ than nonaged oocytes for at least 72 hr (all three 24-hr broods tested). For these experiments, we performed an additional cross with the recovered diplo-X females that allowed us to determine the recombinational history of missegregating chromosomes. Of the 45 diplo-X progeny from aged ord8/ord10 oocytes that we were able to genotype, only 2 arose from missegregation of recombinant chromosomes. In nonaged oocytes, recombinant chromosomes missegregated at a similar frequency (3/47). Therefore, the ord8 allele disrupts the fidelity of achiasmate chromosome segregation, and this effect is enhanced by the aging regimen.
Figure 4.—
Age-dependent NDJ in ord8/ord10 oocytes. Each bar denotes the percentage of X chromosome NDJ in aged (shaded) or nonaged (open) oocytes. Error bars represent 95% confidence intervals. In ord8/ord10 oocytes, normal X chromosomes exhibit age-dependent NDJ in all three broods tested (•••P = 0.0004, ••P = 0.0001, •P = 0.0002; N > 1900 for each brood). The raw data is presented in supplemental Table S3. These data contrast sharply with those observed for ord4/ord10 oocytes (see supplemental Figure S1A).
By monitoring a centromere-linked marker (car), we also determined that the majority of NDJ events were reductional in both aged and nonaged ord8/ord10 oocytes. However, compared to ord4/ord10 oocytes, equational exceptions were more frequent in ord8/ord10 oocytes (12/45 for aged, 5/47 for nonaged). These data indicate that the centromeric function of ORD8 protein is more severely disrupted than that of ORD4 and that complete loss of centromeric cohesion between sister chromatids in ord8/ord10 oocytes increases with age. However, the majority of NDJ events in ord8/ord10 oocytes arise because achiasmate homologs missegregate during the first meiotic division.
Homologous exchange is reduced to a similar degree by ord4 and ord8 mutations:
Why are normal X chromosomes vulnerable to age-dependent NDJ in ord8/ord10 oocytes but not ord4/ord10 oocytes? Because ORD function is required for normal levels of interhomolog exchange (Mason 1976; Miyazaki and Orr-Weaver 1992; Bickel et al. 1997; Webber et al. 2004), we reasoned that the stronger ord8 mutation might suppress crossovers to a greater extent than ord4. If this were the case, ord8/ord10 oocytes would contain more achiasmate X chromosomes than ord4/ord10 oocytes and these would be vulnerable to age-dependent NDJ even in the absence of the FM7a balancer. Similarly, if crossovers between normal X chromosomes were more abundant in ord4/ord10 oocytes than in ord8/ord10 oocytes, we might require a balancer chromosome (that completely suppresses recombination) to elicit age-dependent NDJ of achiasmate chromosomes in ord4/ord10 oocytes. Therefore, we measured X chromosome exchange in the two genotypes to determine if achiasmate bivalents were more prevalent in ord8/ord10 oocytes.
Interestingly, our analysis of X chromosome recombination revealed that crossovers are similarly and substantially reduced in ord4/ord10 and ord8/ord10 oocytes (Table 1). In both genotypes, the total map distance for the X chromosome was reduced to ≤20% of wild type (Table 1). The tetrad exchange rank of bivalents can be inferred from the recombinant and nonrecombinant meiotic products (Weinstein 1936). In wild-type Drosophila oocytes, 6–12% of normal X chromosome bivalents fail to recombine and therefore belong to the E0 tetrad exchange rank (Ashburner 1989; Hawley et al. 1993; Zwick et al. 1999). From our recombination analysis, we estimate the achiasmate X chromosome tetrads (E0) to be ≥79% in both ord4/ord10 and ord8/ord10 oocytes. A smaller scale analysis of recombination on the left arm of chromosome 3 indicated that both alleles also depress autosomal exchange (data not shown).
TABLE 1.
ord4 and ord8 depress meiotic exchange to a similar degree
| Crossovers (cM)
|
Total map distance (cM) | Exchange rank
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | n | sc-cv | cv-v | v-f | f-car | E0 | E1 | E2 | E3 | |
| +/+ | 971 | 11.8 | 20.3 | 18.3 | 6.3 | 56.8 | 9.6 | 69.0 | 19.7 | 1.7 |
| ord4/10 | 610 | 0.7 | 1.6 | 4.6 | 1.8 | 8.7 | 84.5 | 13.5 | 2.0 | 0.0 |
| ord8/10 | 1287 | 1.6 | 2.0 | 4.8 | 2.6 | 11.0 | 79.3 | 19.5 | 1.2 | 0.0 |
Although the majority of the X chromosomes are achiasmate in both ord4/ord10 and ord8/ord10 oocytes, age-dependent NDJ depends on the presence of the FM7a balancer in ord4/ord10 oocytes but not ord8/ord10 oocytes. This suggests that FM7a/X bivalents in ord4/ord10 oocytes are prone to higher levels of NDJ after aging not because FM7a suppresses crossovers, but because of some other attribute of the FM7a chromosome.
Reduced centromere-proximal heterochromatin contributes to age-dependent NDJ of achiasmate chromosomes in ord4/ord10 oocytes:
In addition to promoting arm cohesion and meiotic exchange, ORD also functions at the centromere and is highly enriched at pericentric heterochromatin (see Figure 1). Homologous pairing of centromere proximal heterochromatin is essential for proper achiasmate chromosome disjunction in Drosophila oocytes (Hawley et al. 1993; Hawley and Theurkauf 1993; Karpen et al. 1996) and this pairing is maintained until anaphase I (Dernburg et al. 1996; Gilliland et al. 2009; Hughes et al. 2009). One possibility is that the FM7a chromosome is more vulnerable to increased NDJ in aged ord4/ord10 oocytes because a large portion of the pericentric heterochromatin is displaced to the distal end of the chromosome (Figure 3B). Although FM7a associates efficiently with its partner under normal conditions (Dernburg et al. 1996), the small proximal region of heterochromatin on FM7a may result in homologous pairing that is less resistant to the effects of aging when ORD activity is compromised.
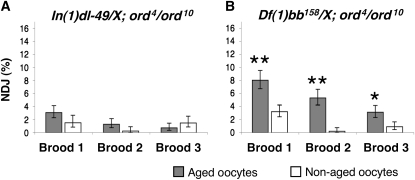
To test the hypothesis that FM7a alters disjunction patterns in aged ord4/ord10 oocytes because centromere-proximal heterochromatin is reduced, we examined the effect of aging on the meiotic segregation of two different X chromosomes in ord oocytes. One X chromosome [In(1)dl-49] contains a large inversion in the euchromatin that significantly suppresses meiotic exchange whereas the other X chromosome [Df(1)bb158] harbors a deletion that removes a large portion of centromere-proximal heterochromatin (see Figure 3, C and D). We subjected ord4/ord10 females containing one normal X chromosome and one of the above “tester” chromosomes to the aging regimen and measured meiotic nondisjunction.
The large inversion on the In(1)dl-49 chromosome (Figure 3C) strongly suppresses recombination with a normal X chromosome (<22% of wild type), and 81% of the tetrads in In(1)dl-49/X oocytes are estimated to be achiasmate (Sturtevant and Beadle 1936; Grell 1962; Roberts 1962). Exchange between In(1)dl-49 and a normal X chromosome will be reduced even further in an ord4/ord10 background, such that the number of achiasmate X chromosomes will approach that achieved with the FM7a balancer. Despite this euchromatic rearrangement, centromere-proximal heterochromatin is unaffected in In(1)dl-49 (Xiang and Hawley 2006). Therefore, this chromosome can be utilized to test whether achiasmate chromosomes with normal heterochromatin are sensitive to age effects in ord4/ord10 oocytes. When In(1)dl-49/X; ord4/ord10 females were subjected to the aging regimen, age-dependent NDJ was not observed (Figure 5A, supplemental Table S4). This result argues that a further reduction of meiotic exchange caused by euchromatic aberrations on the FM7a balancer chromosome is not responsible for the age-dependent NDJ in FM7a/X; ord4/ord10 oocytes.
Figure 5.—
Reduction of centromere-proximal heterochromatin renders X chromosomes vulnerable to age-dependent NDJ in ord4/ord10 oocytes The percentage of X chromosome NDJ is shown for aged (shaded bars) and nonaged (open bars) oocytes. Error bars represent 95% confidence intervals. (A) Aging does not increase X chromosome NDJ In(1)dl-49/y;ord4/10 oocytes (P ≥ 0.0656; N > 790 for each brood). (B) Df(1)bb158/y;ord4/10 oocytes exhibit age dependent in all three broods tested (**P = 0.0001, *P = 0.0059; N > 1030 for each brood). The raw data for A and B are presented in supplemental Tables S4 and S5, respectively.
Unlike In(1)dl-49, the Df(1)bb158 chromosome lacks ∼80% of the pericentric heterochromatin (including the rDNA cluster) (Figure 3D), but contains no aberrations in the euchromatin (Yamamoto and Miklos 1977, 1978). X chromosome meiotic exchange and segregation in Df(1)bb158/X females are similar to wild type (Yamamoto and Miklos 1977, 1978). However, in ord4/ord10 oocytes, a significant percentage of Df(1)bb158/X bivalents will be achiasmate. Following the aging regimen, the Df(1)bb158/X bivalents exhibit significantly higher missegregation in aged ord4/ord10 oocytes compared to the nonaged oocytes of the same genotype (Figure 5B, supplemental Table S5). However, when ORD function was wild type, we did not observe age-dependent NDJ of Df(1)bb158/X chromosomes (supplemental Table S6). The above data indicate that the combination of compromised ORD activity and reduced levels of centromere-proximal heterochromatin act together to increase missegregation of achiasmate chromosomes in aged oocytes.
ORD promotes heterochromatin-mediated pairing:
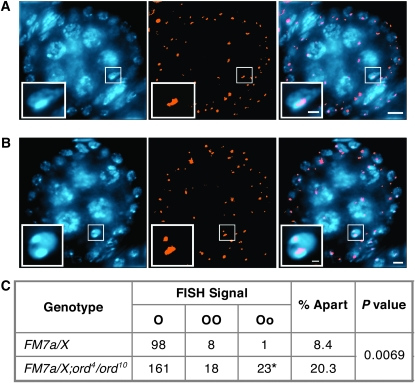
The finding that mutations in ord sensitize achiasmate chromosomes to missegregate in aged oocytes led us to test the hypothesis that ORD activity promotes heterochromatin-mediated association of homologous chromosomes. ORD protein is highly enriched within the pericentric heterochromatin of oocyte chromosomes during prophase I (Webber et al. 2004; Khetani and Bickel 2007) and therefore is in the right place at the right time to assist in the pairing of homologs via pericentric heterochromatin (see Figure 1). To test this possibility, we utilized FISH (fluorescence in situ hybridization) to determine if the association of FM7a with a normal X chromosome is affected in ord mutant oocytes. Cytological analysis has indicated that in wild-type oocytes, both the small region of pericentric heterochromatin and the distal heterochromatin on the FM7a chromosome are able to pair with the centromere-proximal heterochromatin on the normal X chromosome (Dernburg et al. 1996).
For the FISH analysis, we used a probe that recognizes a 359-bp repeat sequence within the pericentric heterochromatin of the X chromosome. In FM7a/X oocytes, hybridization with this probe will result in one spot if the distal and proximal heterochromatin of FM7a both pair with the homologous pericentric sequence on the normal X (Figure 6A) (Dernburg et al. 1996). Conversely, more than one spot will be observed (Figure 6B) if either the distal or the proximal heterochromatin of FM7a is not paired with the normal X homolog (Dernburg et al. 1996). Furthermore, because the centromere proximal heterochromatin of FM7a is much smaller than the distal heterochromatin, a small FISH signal separated from a large FISH signal indicates that the centromeres of the two X chromosomes are no longer paired (see Figure 6B).
Figure 6.—
Disruption of ord activity weakens FM7a/X heterochromatic pairing. (A and B) Drosophila ovaries from females reared under normal conditions (no aging regimen) were hybridized with the 359-bp repeat pericentric heterochromatin probe (orange) and stained with DAPI (blue). Single egg chambers are shown with the oocyte nucleus enlarged in the inset. Bars, 5 μm in the panels; 1 μm in the insets. (A) Pairing of the distal and the proximal heterochromatin of FM7a with the pericentric heterochromatin of the normal X chromosome results in a single focus within the oocyte nucleus. A stage 3 oocyte is shown. (B) Separated FISH signals result if the interrupted heterochromatin of FM7a is not completely paired with its homolog. A stage 5 oocyte is shown. (C) Heterochromatin pairing was quantified in FM7a/X; ord+ and FM7a/X; ord4/ord10 oocytes using the 359-bp FISH probe. The data represent tabulated results for oocyte stages 2–11 (see supplemental Table S7). “O” denotes a single FISH signal (as seen in A). “OO” denotes separated FISH signals of equal size that occur when the distal heterochromatin of FM7a is not paired with the normal X chromosome. “Oo” denotes separated FISH signals of different sizes (as shown in B) that occur when the centromere-proximal heterochromatin of FM7a fails to pair with the normal X chromosome. (*) 2/23 oocytes contained three FISH signals. Pairing between the heterochromatic regions of the FM7a balancer and a normal X chromosome is disrupted more often in ord4/ord10 than in ord+ oocytes (P = 0.0069).
For these experiments, we examined prophase I oocytes spanning ovariole stages 2–11 (see Figure 2C). Stages 2–6 represent pachytene oocytes and stages 7–11 correspond to oocytes after synaptonemal complex disassembly but before nuclear envelope breakdown or spindle formation (Ashburner 1989). Although pairing between homologous euchromatic regions is lost at the end of pachytene (Dernburg et al. 1996), the pericentric heterochromatin of homologs remains in contact until anaphase I (Dernburg et al. 1996; Gilliland et al. 2009; Hughes et al. 2009). Therefore, analysis of FM7a/X heterochromatin pairing during stages 2–11 allowed us to examine the behavior of this achiasmate bivalent during pachytene and postpachytene stages.
FISH analysis demonstrated that heterochromatin pairing between the FM7a balancer and a normal X chromosome is significantly disrupted in ord oocytes. In ord+ oocytes, the 359-bp satellite sequences were apart (two spots) in 8.4% of the oocytes examined (Figure 6C, supplemental Table S7). In addition, only one out of the nine instances observed corresponded to separated centromeres (Oo). In the ord4/ord10 mutant oocytes, disruption of heterochromatin pairing was significantly more frequent (P = 0.0069). Separated FISH signals were observed in 20.3% of the ord oocytes (Figure 6C, supplemental Table S7) and detectable at every stage examined (stages 2–11). Moreover, centromeres of FM7a and the normal X chromosome were apart in 23 out of the 202 oocytes examined, more than 10 times the incidence that we observed for wild type. The separated FISH signals in FM7a/X;ord4/ord10 oocytes do not represent individual sister chromatids because in genetic tests, this same genotype results in very few diplo-X progeny that arise from an equational NDJ event (see above). Therefore, our FISH data indicate that pairing at the centromere-proximal heterochromatin in FM7a/X bivalents is compromised in ord mutants (even in the absence of aging) and supports the model that the cohesion protein ORD promotes heterochromatin-mediated association of achiasmate bivalents.
DISCUSSION
In general, proper segregation of homologous chromosomes during meiosis requires the formation and maintenance of chiasmata (Buonomo et al. 2000; Bickel et al. 2002; Hodges et al. 2005). Cohesion along the arms of sister chromatids is required to hold recombinant homologs together until anaphase I (see Figure 1). In some organisms, the presence of an achiasmate pathway ensures that bivalents that lack a crossover still segregate accurately (Wolf 1994). In Drosophila oocytes, accurate segregation of achiasmate chromosomes relies on homologous pairing of centromere-proximal heterochromatin (see Figure 1). Although heterochromatin-mediated association of all homologs occurs (Dernburg et al. 1996), only achiasmate bivalents require this mechanism to segregate properly (see Figure 1). Our previous work suggested an unexpected link between sister-chromatid cohesion and the achiasmate segregation pathway (Jeffreys et al. 2003) but how cohesion proteins are able to influence the disjunction of achiasmate chromosomes was not readily apparent. In this study we have used mutations that disrupt the cohesion protein ORD and X chromosomes with different amounts of centromere proximal heterochromatin to better understand how sister-chromatid cohesion contributes to achiasmate segregation.
In all organisms examined, defects in meiotic sister-chromatid cohesion also have a severe effect on meiotic exchange (van Heemst and Heyting 2000). This holds true for ordnull oocytes in which meiotic crossing over on the X chromosome is reduced to ∼16% of wild type (Bickel et al. 1997). Surprisingly, we have found that ord mutations that only mildly affect segregation (ord4 and ord8), exhibit a severe defect in meiotic recombination; nonexchange tetrads in ord4/ord10 and ord8/ord10 oocytes are as frequent as when ORD activity is absent. This differential effect on meiotic exchange and segregation may arise because arm cohesion is disrupted to a greater degree than centromeric cohesion in both ord4 and ord8 mutant oocytes. Alternatively, it is also possible that a small defect in arm cohesion has a substantial impact on meiotic exchange.
Studies using both flies and mice support the hypothesis that sister-chromatid cohesion deteriorates as oocytes age and results in the missegregation of recombinant chromosomes (Hodges et al. 2005; Subramanian and Bickel 2008). In addition, we find that aging in Drosophila oocytes is accompanied by a significant increase in the missegregation of achiasmate bivalents when oocytes begin with meiotic cohesion that is slightly compromised (Jeffreys et al. 2003; this study). Our ability to examine the effect of age on the fidelity of meiotic chromosome segregation in Drosophila oocytes has provided us with a sensitive assay that has uncovered a functional link between meiotic cohesion and pericentric heterochromatin in the pairing and disjunction of achiasmate homologs. We observe age-dependent NDJ of achiasmate chromosomes in ord4/ord10 as well as ord8/ord10 mutant oocytes. However, in the presence of the weaker ord4 allele, achiasmate bivalents are only susceptible to age effects when the centromere-proximal heterochromatin of one of the X chromosomes is also reduced. The effect we observe depends on reduction of ORD activity; age-dependent NDJ of FM7a/X achiasmate bivalents does not occur in ord+ oocytes (Jeffreys et al. 2003).
Using in situ hybridization, we have shown that pairing between the heterochromatin of FM7a and a normal X chromosome is destabilized in ord mutant oocytes. In our previous FISH analyses of ordnull oocytes we did not detect pairing defects between two normal X chromosomes during pachytene (Webber et al. 2004) or prior to nuclear envelope breakdown in late prophase (Bickel et al. 2002). However, the reduction of centromere proximal heterochromatin on FM7a provides a sensitized system that has revealed a role for ORD in promoting the continued physical association between the centromere-proximal heterochromatin of homologous chromosomes.
Consistent with a role for cohesion proteins in heterochromatin-mediated homolog pairing, ORD, SMC1, and SMC3 are enriched at the centric/pericentric regions of meiotic chromosomes in Drosophila oocytes (Webber et al. 2004; Khetani and Bickel 2007). This localization pattern is not unique to Drosophila meiosis. Strong pericentric localization of cohesin subunits has been observed during both mitosis and meiosis in several organisms (Watanabe and Kitajima 2005). Moreover, in S. pombe, recruitment of cohesins to the pericentric heterochromatin depends on Swi6 (HP1 ortholog) and therefore on heterochromatin structure (Bernard et al. 2001; Bernard and Allshire 2002; Nonaka et al. 2002). However, whether such a mechanism operates universally is controversial (Koch et al. 2008).
Here we show that the sister-chromatid cohesion protein ORD plays a role in maintaining heterochromatin-mediated associations between achiasmate homologs and that these associations weaken with age. Moreover, our recent work also indicates that when the dosage of the cohesin subunit SMC1 is reduced, missegregation of nonrecombinant chromosomes increases with age (Subramanian and Bickel 2008). Therefore, two different proteins required for meiotic cohesion in Drosophila oocytes participate in the accurate segregation of achiasmate chromosomes. These data argue that ORD and cohesin proteins function similarly to ensure the proper segregation of achiasmate homologs.
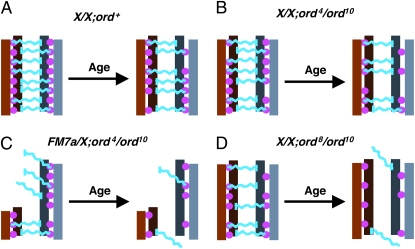
We propose that cohesion proteins within pericentric heterochromatin provide a structural framework necessary for the physical association of homologous chromosomes (Figure 7). One possibility is that additional (as yet unidentified) proteins interact with cohesion proteins and heterochromatin proteins to form a bridge between the homologous chromosomes (Figure 7A). A more conservative possibility is that a heterochromatin protein on one homolog interacts directly with a cohesion protein on the other homolog. Such models are consistent with the recent report of Hawley and colleagues describing heterochromatic threads connecting achiasmate bivalents during prometaphase I oscillation (Hughes et al. 2009). We propose that when oocytes age under normal conditions (i.e., wild-type sister-chromatid cohesion as well as a normal amount of centromere-proximal heterochromatin), achiasmate segregation errors are not observed because the association of homologs remains intact even though some cohesion is lost with age (Figure 7A). Similarly, even when cohesion is weakened by the ord4 mutation, if a large region of heterochromatin resides close to the centromere (normal X chromosome), the compromised activity of ORD4 protein is still sufficient to support pairing between homologous chromosomes when oocytes undergo aging (Figure 7B). However, when the number of heterochromatin pairing sites near the centromere is reduced [FM7a or Df(1)bb158] and the strength of these homolog connections is also compromised by the partial loss-of-function ord4 mutation, loss of cohesion with age causes achiasmate chromosomes to lose their associations and exhibit age-dependent NDJ (Figure 7C). In ord8/ord10 mutant flies, ORD activity at the centromere is more severely compromised than in ord4/ord10 oocytes (Figure 7D) and we observe age-dependent NDJ of X chromosomes that contain the normal amount of centromere-proximal heterochromatin. Our data suggest that even though the amount of heterochromatin is not reduced, the physical connections holding achiasmate homologs together in ord8/ord10 oocytes are weaker and exhibit a greater susceptibility to age. In some cases, centromeric cohesion is completely lost in ord8/ord10 oocytes (not depicted), resulting in equational NDJ events and these also become more frequent as oocytes age.
Figure 7.—
Model for how cohesion proteins and pericentric heterochromatin might cooperate to maintain the association of achiasmate homologs. This schematic provides one possible model to explain why heterochromatin-mediated attachments between achiasmate chromosomes depend on sister-chromatid cohesion proteins and how decline of cohesion with age contributes to achiasmate nondisunction. The pericentric heterochromatin of a set of achiasmate homologs is depicted in shades of brown and gray, with sister chromatids shown in different shades. Pink filled circles represent sister-chromatid cohesion proteins. In this model, hypothetical linker proteins that physically connect the two homologs are depicted in blue. Each blue “linker” physically interacts with heterochromatin of one homolog and cohesion proteins that join the two sisters of the other homolog. For simplicity, interaction of the linkers with heterochromatin (vertical blue bars) is shown for only one of the sisters. A more conservative possibility is that the blue “linker” represents a heterochromatin protein on one homolog that interacts with a cohesion protein on the other homolog. (A) In wild-type flies with two normal X chromosomes, even though some deterioration of cohesion occurs during the aging process, the association of achiasmate chromosomes remains intact. (B) In ord4/ord10 oocytes, the centromeric function of ORD is slightly compromised even in young oocytes (fewer filled circles). However, weakening of cohesion with age does not significantly affect the association between homologs when normal amounts of heterochromatin reside near the centromere. (C) In contrast, when centromere-proximal heterochromatin is reduced (FM7a) in ord4/ord10 oocytes, the combination of reduced ORD activity at the centromere, fewer heterochromatin attachment sites and deterioration of cohesion with age causes achiasmate homologs to lose their association. (For ease of illustration, the reduction of heterochromain in FM7a is not drawn to scale). (D) In ord8/ord10 oocytes, the centromeric function of ORD is more severely compromised and age-dependent deterioration of cohesion significantly affects homolog association even when pericentric heterochromatin is normal. Moreover, sister chromatid NDJ is also observed with the stronger ord8 mutation and the frequency of equational NDJ increases when oocytes undergo aging. Note that the loss of homolog association shown in C and D is meant to represent a significant increase in achiasmate missegregation with age, not complete dissociation of the bivalents in 100% of the oocytes.
In the model presented in Figure 7, cohesion proteins and heterochromatin both interact directly with hypothetical proteins that bridge the homologous chromosomes. Another possibility (not depicted) is that cohesion proteins within the pericentric heterochromatin on the two homologs physically interact with each other and form the bridge themselves. Such a scenario would be consistent with the “hand-cuff” model recently described in which individual cohesin rings on two chromatids are held together by a common Scc3/SA subunit (Zhang et al. 2008). A third alternative (also not depicted) is that cohesion proteins associated with the centromere-proximal heterochromatin play a more indirect role in which they help maintain proper heterochromatin structure, which is required for achiasmate associations but cohesion proteins do not interact directly with the proteins that physically hold the achiasmate homologs.
Although at this time we cannot distinguish between the above scenarios, our findings clearly indicate that cohesion proteins play an important role in heterochromatin-mediated pairing and accurate segregation of achiasmate chromosomes in Drosophila oocytes. Notably, our data argue that meiotic cohesion proteins not only function to keep sister chromatids associated and chiasmate bivalents intact, but also collaborate with heterochromatin to keep achiasmate chromosomes physically connected, thereby ensuring their proper segregation. Although each set of homologs exhibits heterochromatin-mediated association during prophase I (Dernburg et al. 1996), only achiasmate bivalents require this mechanism to segregate properly (see Figure 1). Because the majority of bivalents are achiasmate in ord mutant oocytes, our analysis and model are restricted to their behavior in this article. However, given its essential role in arm cohesion and chiasma maintenance (Bickel et al. 2002), we expect that deterioration of ORD activity during the aging process most likely also reduces chiasmata stability.
Our work with Drosophila oocytes that have been subjected to aging indicates that both chiasmate and achiasmate bivalents are impacted by the aging process (Jeffreys et al. 2003; Subramanian and Bickel 2008; this study). In our studies, the unifying factor for age-dependent NDJ of chiasmate and achiasmate chromosomes is loss of cohesion with age. Deterioration of arm cohesion with age leads to destabilization of chiasmata, which allows recombinant homologs to missegregate more frequently in aged oocytes (Subramanian and Bickel 2008). In addition, the activity of cohesion proteins enriched at pericentric heterochromatin also appears to decline with age (this article) and this leads to increased missegregation of achiasmate chromosomes.
Chromosome segregation errors in human oocytes increase dramatically as women age and much work has focused on understanding the mechanisms that lead to age-dependent NDJ of recombinant chromosomes (Sherman et al. 1994; Lamb et al. 1997, 2005; Hassold and Hunt 2001; Hodges et al. 2005). Given that chiasmata must remain intact for decades in human oocytes, loss of cohesion with age could account, at least in part, for the high incidence of NDJ of chiasmate bivalents observed in older women and studies in mice and Drosophila support this conclusion (Hodges et al. 2005; Subramanian and Bickel 2008). Whether an achiasmate chromosome segregation pathway exists in human oocytes remains controversial (Koehler and Hassold 1998). However, recent analysis of the recombinational history of chromosome 21 in human oocytes indicates that achiasmate chromosomes segregate accurately more often than expected; 20% of the normal segregation events analyzed originated from achiasmate chromosome 21 tetrads (Oliver et al. 2008). Moreover, the data of Sherman and colleagues also suggest that a greater proportion of E0 bivalents missegregate in older oocytes than in the younger age groups (Oliver et al. 2008). Although still speculative, such evidence supports the hypothesis that an achiasmate pathway may indeed operate during female meiosis in humans and that its effectiveness deteriorates with age. While additional evidence will be necessary to support this claim, the link between sister-chromatid cohesion and achiasmate segregation that we have uncovered in Drosophila oocytes provides an intriguing framework within which to consider the maternal age effect in humans.
Acknowledgments
We thank Mark Borsuk for help with the statistical analyses and members of the Bickel lab for helpful discussions and suggestions. We are grateful to the Bloomington stock center for providing several of the fly stocks used in this study. This work was funded by a National Institutes of Health (GM-59354) award (to S.E.B.).
References
- Agresti, A., and B. A. Coull, 1998. Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 52 119–126. [Google Scholar]
- Ashburner, M., 1989. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Balicky, E. M., M. W. Endres, C. Lai and S. E. Bickel, 2002. Meiotic cohesion requires accumulation of ORD on chromosomes prior to condensation. Mol. Biol. Cell 21 3890–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, P., and R. Allshire, 2002. Centromeres become unstuck without heterochromatin. Trends Cell Biol. 12 419–424. [DOI] [PubMed] [Google Scholar]
- Bernard, P., J. F. Maure, J. F. Partridge, S. Genier, J. P. Javerzat et al., 2001. Requirement of heterochromatin for cohesion at centromeres. Science 294 2539–2542. [DOI] [PubMed] [Google Scholar]
- Bickel, S. E., T. Orr-Weaver and E. M. Balicky, 2002. The sister-chromatid cohesion protein ORD is required for chiasma maintenance in Drosophila oocytes. Curr. Biol. 12 925–929. [DOI] [PubMed] [Google Scholar]
- Bickel, S. E., D. W. Wyman, W. Y. Miyazaki, D. P. Moore and T. L. Orr-Weaver, 1996. Identification of ORD, a Drosophila protein essential for sister-chromatid cohesion. EMBO J. 15 1451–1459. [PMC free article] [PubMed] [Google Scholar]
- Bickel, S. E., D. W. Wyman and T. L. Orr-Weaver, 1997. Mutational analysis of the Drosophila sister-chromatid cohesion protein ORD and its role in the maintenance of centromeric cohesion. Genetics 146 1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo, S. B., R. K. Clyne, J. Fuchs, J. Loidl, F. Uhlmann et al., 2000. Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 103 387–398. [DOI] [PubMed] [Google Scholar]
- Cheslock, P. S., B. J. Kemp, R. M. Boumil and D. S. Dawson, 2005. The roles of MAD1, MAD2 and MAD3 in meiotic progression and the segregation of nonexchange chromosomes. Nat. Genet. 37 756–760. [DOI] [PubMed] [Google Scholar]
- Davis, L., and G. R. Smith, 2005. Dynein promotes achiasmate segregation in Schizosaccharomyces pombe. Genetics 170 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, D. S., A. W. Murray and J. W. Szostak, 1986. An alternate pathway for meiotic chromosome segregation in yeast. Science 234 713–717. [DOI] [PubMed] [Google Scholar]
- Dernburg, A. F., J. W. Sedat and R. S. Hawley, 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86 135–146. [DOI] [PubMed] [Google Scholar]
- Ding, D. Q., A. Yamamoto, T. Haraguchi and Y. Hiraoka, 2004. Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev. Cell 6 329–341. [DOI] [PubMed] [Google Scholar]
- Doll, E., M. Molnar, Y. Hiraoka and J. Kohli, 2005. Characterization of rec15, an early meiotic recombination gene in Schizosaccharomyces pombe. Curr. Genet. 48 323–333. [DOI] [PubMed] [Google Scholar]
- Gilliland, W. D., S. F. Hughes, D. R. Vietti and R. S. Hawley, 2009. Congression of achiasmate chromosomes to the metaphase plate in Drosophila melanogaster oocytes. Dev. Biol. 325 122–128. [DOI] [PubMed] [Google Scholar]
- Grell, R. F., 1962. A new model for secondary nondisjunction: the role of distributive pairing. Genetics 47 1737–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guacci, V., and D. B. Kaback, 1991. Distributive disjunction of authentic chromosomes in Saccharomyces cerevisiae. Genetics 127 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold, T., and P. Hunt, 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2 280–291. [DOI] [PubMed] [Google Scholar]
- Hawley, R. S., H. Irick, A. E. Zitron, D. A. Haddox, A. Lohe et al., 1993. There are two mechanisms of achiasmate segregation in Drosophila, one of which requires heterochromatic homology. Dev. Genet. 13 440–467. [DOI] [PubMed] [Google Scholar]
- Hawley, R. S., and W. E. Theurkauf, 1993. Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet. 9 310–317. [DOI] [PubMed] [Google Scholar]
- Hodges, C. A., E. Revenkova, R. Jessberger, T. J. Hassold and P. A. Hunt, 2005. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat. Genet. 37 1351–1355. [DOI] [PubMed] [Google Scholar]
- Hughes, S. E., W. D. Gilliland, J. L. Cotitta, S. Takeo, K. A. Collins et al., 2009. Heterochromatic threads connect oscillating chromosomes during prometaphase I in Drosophila oocytes. PLoS Genet. 5: e1000348. [DOI] [PMC free article] [PubMed]
- Jeffreys, C. A., P. S. Burrage and S. E. Bickel, 2003. A model system for increased meiotic nondisjunction in older oocytes. Curr. Biol. 13 498–503. [DOI] [PubMed] [Google Scholar]
- Karpen, G. H., M. H. Le and H. Le, 1996. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science 273 118–122. [DOI] [PubMed] [Google Scholar]
- Kemp, B., R. M. Boumil, M. N. Stewart and D. S. Dawson, 2004. A role for centromere pairing in meiotic chromosome segregation. Genes Dev. 18 1946–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetani, R. S., and S. E. Bickel, 2007. Regulation of meiotic cohesion and chromosome core morphogenesis during pachytene in Drosophila oocytes. J. Cell. Sci. 120 3123–3137. [DOI] [PubMed] [Google Scholar]
- King, R. C., 1957. Oogenesis in adult Drosophila melanogaster. II. Stage distribution as a function of age. Growth 21 95–102. [PubMed] [Google Scholar]
- Koch, B., S. Kueng, C. Ruckenbauer, K. S. Wendt and J. M. Peters, 2008. The Suv39h-HP1 histone methylation pathway is dispensable for enrichment and protection of cohesin at centromeres in mammalian cells. Chromosoma 117 199–210. [DOI] [PubMed] [Google Scholar]
- Koehler, K. E., and T. J. Hassold, 1998. Human aneuploidy: lessons from achiasmate segregation in Drosophila melanogaster. Ann. Hum. Genet. 62 467–479. [DOI] [PubMed] [Google Scholar]
- Kudo, N. R., K. Wassmann, M. Anger, M. Schuh, K. G. Wirth et al., 2006. Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell 126 135–146. [DOI] [PubMed] [Google Scholar]
- Lamb, N. E., E. Feingold, A. Savage, D. Avramopoulos, S. Freeman et al., 1997. Characterization of susceptible chiasma configurations that increase the risk for maternal nondisjunction of chromosome 21. Hum. Mol. Genet. 6 1391–1399. [DOI] [PubMed] [Google Scholar]
- Lamb, N. E., S. L. Sherman and T. J. Hassold, 2005. Effect of meiotic recombination on the production of aneuploid gametes in humans. Cytogenet. Genome Res. 111 250–255. [DOI] [PubMed] [Google Scholar]
- Lee, J. Y., and T. L. Orr-Weaver, 2001. The molecular basis of sister-chromatid cohesion. Annu. Rev. Cell Dev. Biol. 17 753–777. [DOI] [PubMed] [Google Scholar]
- Mason, J. M., 1976. Orientation disruptor (ord): a recombination-defective and disjunction-defective meiotic mutant in Drosophila melanogaster. Genetics 84 545–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneely, P. M., A. F. Farago and T. M. Kauffman, 2002. Crossover distribution and high interference for both the X chromosome and an autosome during oogenesis and spermatogenesis in Caenorhabditis elegans. Genetics 162 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, W. Y., and T. L. Orr-Weaver, 1992. Sister-chromatid misbehavior in Drosophila ord mutants. Genetics 132 1047–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, W. Y., and T. L. Orr-Weaver, 1994. Sister-chromatid cohesion in mitosis and meiosis. Annu. Rev. Genet. 28 167–187. [DOI] [PubMed] [Google Scholar]
- Molnar, M., J. Bahler, J. Kohli and Y. Hiraoka, 2001. Live observation of fission yeast meiosis in recombination-deficient mutants: a study on achiasmate chromosome segregation. J. Cell. Sci. 114 2843–2853. [DOI] [PubMed] [Google Scholar]
- Nonaka, N., T. Kitajima, S. Yokobayashi, G. Xiao, M. Yamamoto et al., 2002. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 4 89–93. [DOI] [PubMed] [Google Scholar]
- Oliver, T. R., E. Feingold, K. Yu, V. Cheung, S. Tinker et al., 2008. New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet. 4 e1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasierbek, P., M. Jantsch, M. Melcher, A. Schleiffer, D. Schweizer et al., 2001. A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev. 15 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, P., 1962. Interchromosomal effects and the relation between crossing-over and nondisjunction. Genetics 47 1691–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D. N., K. Cant and L. Cooley, 1994. Morphogenesis of Drosophila ovarian ring canals. Development 120 2015–2025. [DOI] [PubMed] [Google Scholar]
- Ross, L. O., S. Rankin, M. F. Shuster and D. S. Dawson, 1996. Effects of homology, size and exchange on the meiotic segregation of model chromosomes in Saccharomyces cerevisiae. Genetics 142 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, S. L., M. B. Petersen, S. B. Freeman, J. Hersey, D. Pettay et al., 1994. Non-disjunction of chromosome 21 in maternal meiosis I: Evidence for a maternal age-dependent mechanism involving reduced recombination. Hum. Mol. Genet. 3 1529–1535. [DOI] [PubMed] [Google Scholar]
- Siomos, M. F., A. Badrinath, P. Pasierbek, D. Livingstone, J. White et al., 2001. Separase is required for chromosome segregation during meiosis I in Caenorhabditis elegans. Curr. Biol. 11 1825–1835. [DOI] [PubMed] [Google Scholar]
- Sturtevant, A. H., and G. W. Beadle, 1936. The relations of inversions in the X chromosome of Drosophila melanogaster to crossing over and disjunction. Genetics 21 554–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, V. V., and S. E. Bickel, 2008. Aging predisposes oocytes to meiotic nondisjunction when the cohesin subunit SMC1 is reduced. PLoS Genet. 4 e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf, W. E., and R. S. Hawley, 1992. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann, F., 2001. Chromosome cohesion and segregation in mitosis and meiosis. Curr. Opin. Cell Biol. 13 754–761. [DOI] [PubMed] [Google Scholar]
- van Heemst, D., and C. Heyting, 2000. Sister chromatid cohesion and recombination in meiosis. Chromosoma 109 10–26. [DOI] [PubMed] [Google Scholar]
- Vazquez, J., A. S. Belmont and J. W. Sedat, 2002. The dynamics of homologous chromosome pairing during male Drosophila meiosis. Curr. Biol. 12 1473–1483. [DOI] [PubMed] [Google Scholar]
- Watanabe, Y., and T. S. Kitajima, 2005. Shugoshin protects cohesin complexes at centromeres. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber, H. A., L. Howard and S. E. Bickel, 2004. The cohesion protein ORD is required for homologue bias during meiotic recombination. J. Cell Biol. 164 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, A., 1936. The theory of multiple-strand crossing over. Genetics 21 155–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, K. W., 1994. How meiotic cells deal with non-exchange chromosomes. BioEssays 16 107–114. [DOI] [PubMed] [Google Scholar]
- Xiang, Y., and R. S. Hawley, 2006. The mechanism of secondary nondisjunction in Drosophila melanogaster females. Genetics 174 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M., and G. L. Miklos, 1977. Genetic dissection of heterochromatin in Drosophila: the role of basal X heterochromatin in meiotic sex chromosome behaviour. Chromosoma 60 283–296. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M., and G. L. Miklos, 1978. Genetic studies on heterochromatin in Drosophila melanogaster and their implications for the functions of satellite DNA. Chromosoma 66 71–98. [DOI] [PubMed] [Google Scholar]
- Zhang, N., S. G. Kuznetsov, S. K. Sharan, K. Li, P. H. Rao et al., 2008. A handcuff model for the cohesin complex. J. Cell Biol. 183 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick, M. E., D. J. Cutler and C. H. Langley, 1999. Classic Weinstein: tetrad analysis, genetic variation and achiasmate segregation in Drosophila and humans. Genetics 152 1615–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]