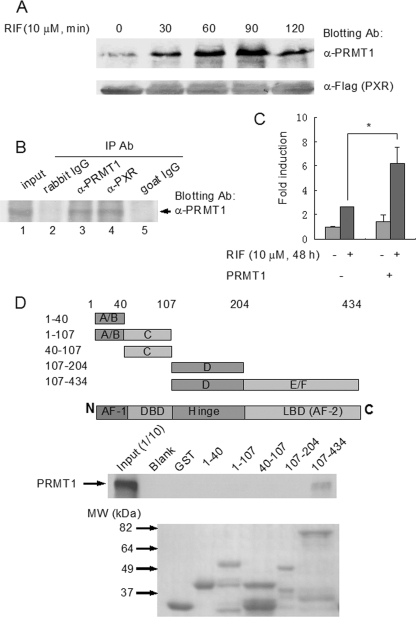
FIGURE 2.
PRMT1 interacts with PXR in a ligand-dependent manner. A, PXR-HepG2 cells were treated with rifampicin (10 μm, 0, 30, 60, 90, 120 min) and subjected to co-immunoprecipitation with anti-FLAG antibody-coupled beads. The precipitates were eluted with 3× FLAG peptide and analyzed by Western blotting with PRMT1 antibody. Anti-FLAG antibody blotting was used to show the equal loading of the samples. B, liver tissue from a VP16-hPXR transgenic mouse was homogenized in the Co-IP lysis buffer and co-immunoprecipitated with goat anti-PXR (lane 4) and rabbit anti-PRMT1 antibodies (lane 3). Goat IgG (lane 5) and rabbit IgG (lane 2) were used as negative controls. 1:10 lysate was loaded as the input control (lane 1). Precipitates were analyzed by Western blotting with PRMT1 antibody. C, CV-1 cells were transfected with the bait plasmid, pBIND-PXR, and the reporter pG5-luc vector, with cotransfection of the prey plasmid pACT-PRMT1 or blank pACT plasmid. Six hours after transfection, cells were treated with rifampicin (10 μm) or vehicle for an additional 48 h. The interaction was characterized by luciferase activity. *, statistically significant difference (t test, p < 0.01). The data are the means ± S.D. of three independent results. D, mapping of the interactive domains of PXR with PRMT1 by GST pull-down assay. Various PXR fragments were fused with GST and the fusion peptides coupled with glutathione-Sepharose beads were incubated with radiolabeled PRMT1. The precipitated complexes were analyzed by autoradiography following SDS-PAGE (middle panel). Upper panel, illustration of PXR fragments. Lower panel, loading control of the GST-fused PXR fragments (Coomassie Blue staining).