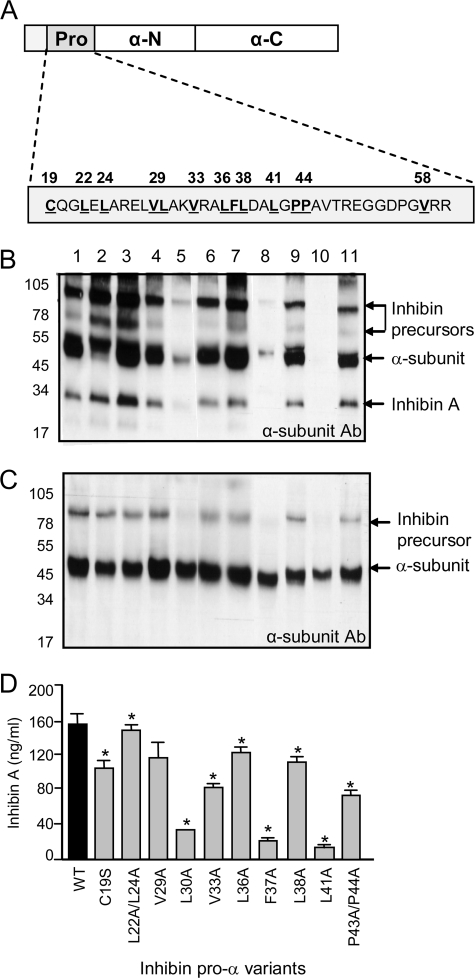
FIGURE 1.
Effects of α-subunit prodomain (Pro) mutations on inhibin A biosynthesis. A, hydrophobic residues in the inhibin α-subunit prodomain were substituted with alanine using in vitro mutagenesis. To determine the effects of amino acid substitutions on inhibin A production, culture medium (B) and cell lysate (C) from CHO cells transfected with either wild type (lane 1) or mutant α-subunit (lanes 2–11), in combination with the βA-subunit, were analyzed by Western blot. Samples were detected with the R1 mAb, specific for the inhibin αC (mature) domain. The 31-kDa inhibin A dimer, 52-kDa free α-subunit, and higher molecular mass inhibin precursors forms (65 and 95 kDa) are noted. D, the effect of α-subunit prodomain mutations on inhibin A expression in CHO culture medium was also determined by ELISA (* = p < 0.05). WT, wild type.