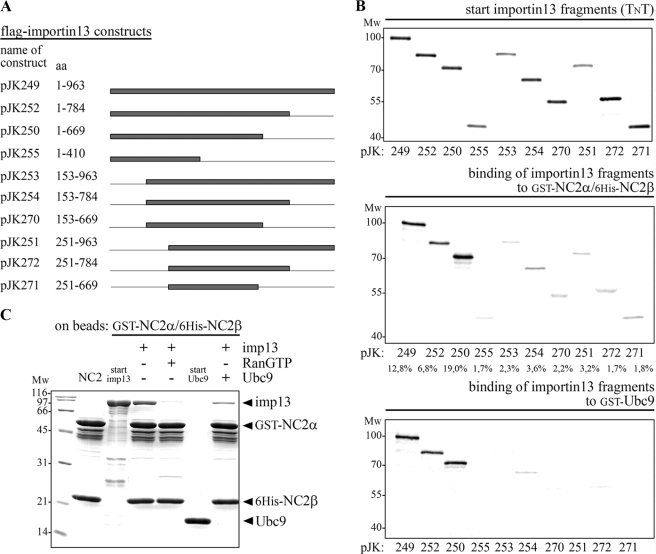
FIGURE 6.
The C terminus of importin 13 is dispensable for recognition of the NC2 complex. A, the names of the importin 13 expression constructs used and the amino acids contained in the constructs are listed. Lines indicate the deleted regions and gray bars represent the different importin 13 fragments. B, the minimal importin 13 fragment that binds to the NC2 complex and UBC9 consists of amino acids 1–669. The two NC2 subunits, GST-NC2α and His6-NC2β, as well as GST-UBC9 were (co-)expressed in E. coli and used as bait after immobilization on glutathione-Sepharose. The immobilized GST-NC2α/His6-NC2β complex and GST-UBC9 were incubated with in vitro transcribed and translated 35S-labeled importin 13 fragments; all from the TnT coupled reticulocyte lysate. Starting material (20% of the importin 13 fragments that were used) and bound fractions were analyzed by SDS-PAGE followed by phosphorimaging (Amersham Biosciences). Among the importin 13 fragments, wild-type importin 13, and amino acids 1–669 of importin 13 showed the highest binding competence for the NC2 complex (see relative binding in percent below the gels, quantified with the program ImageQuant 5.2). A similar binding pattern was observed for UBC9; also here importin 13 fragments lacking the N terminus did not significantly bind to the immobilized substrate. C, the binding sites in importin 13 for the NC2 complex and UBC9 overlap. Importin 13 interacts with the NC2 complex in an UBC9 sensitive manner. Importin 13 was bound specifically to the immobilized GST-NC2α/His6-NC2β complex and this binding was reduced in the presence of equal amounts of UBC9. MW, molecular mass in kilodalton; imp, importin; aa, amino acids.