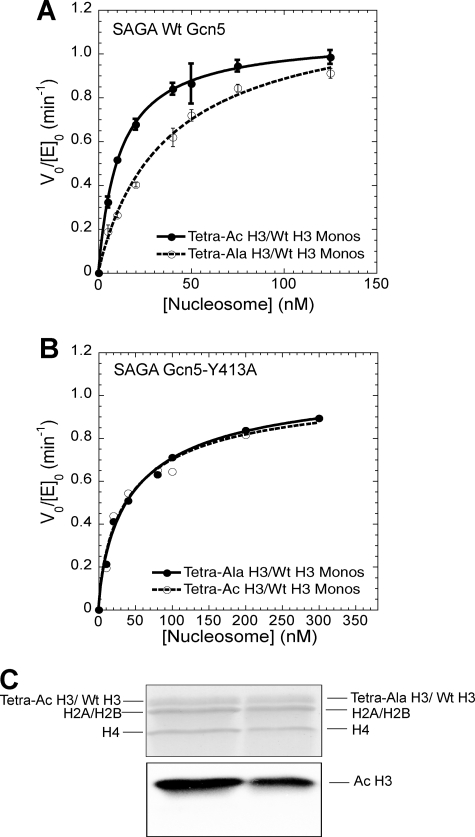
FIGURE 3.
When one of the two H3 tails in a nucleosome is acetylated, the Gcn5 bromodomain promotes acetylation of the other H3 tail. A, a comparison of wild-type SAGA HAT activity on mononucleosomes containing Tetra-Ac H3/WT H3 (solid circles) or Tetra-Ala H3/WT H3 (open circles) octamers. Fitting of the data gives a half-saturation concentration of 11.5 ± 2.4 nm (Tetra-Ac H3/WT H3 nucleosomes, solid line) and 38.9 ± 6.9 nm (Tetra-Ala H3/WT H3 nucleosomes, dashed line). Data were adapted from Li and Shogren-Knaak (17) B, a comparison of HAT activity of SAGA Gcn5-Y413A complex on mononucleosomes containing Tetra-Ac H3/WT H3 (open circles) and Tetra-Ala H3/WT H3 (solid circles) octamers. Two independent experiments were performed at each nucleosome concentration. The half-saturation concentrations obtained from fitting the data are 30.6 nm (Tetra-Ac H3/WT H3 nucleosomes, dashed line) and 32.8 nm (Tetra-Ala H3/WT H3 nucleosomes, solid line). C, a fluorogram showing the histone specificity of SAGA acetylation on mononucleosomes that are either preacetylated or unacetylated on one H3 histone tail (lanes 1 and 2, respectively). The Coomassie Blue-stained gel (upper panel) and corresponding fluorogram (lower panel) are shown.