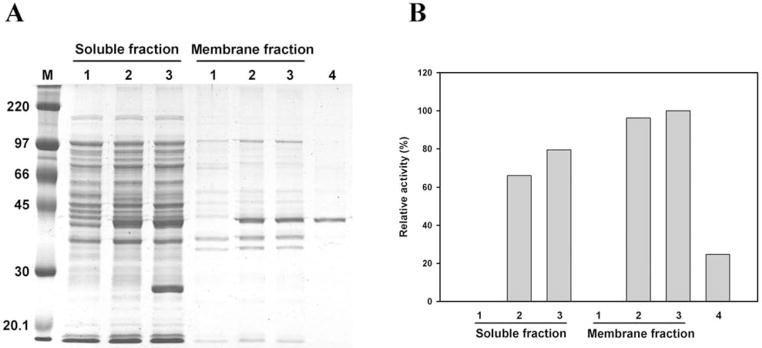
Fig. (6).
Expression and purification of CHLαGcT. (A) SDS-polyacrylamide gel electrophoresis of proteins in both soluble and membrane fractions (lanes 1–3: pTKNd6xH vector only; 2, pTKNd6xH-CHLαGcT; 3, pTKNd6xH-CHLαGcT + pRARE (Novagen), and of protein purified from the soluble fraction using Ni-NTA column (lane 4). (B) Distribution of CHLαGcT activity in samples shown in (A). The amount of protein in lane 4 was approximately one-third of that in lane 3 of the membrane fraction. Adapted from Lee, H.; Kobayashi, M.; Wang, P.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. Biochem. Biophys. Res. Commun., 2006, 349(4), 1235–1241.