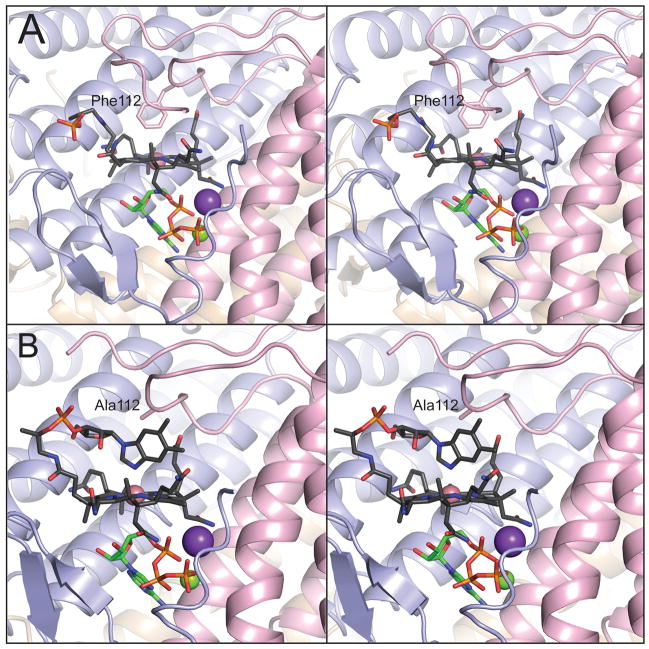
FIGURE 1.
Phe112 displaces the lower ligand of cob(II)alamin in LrPduO to generate a four-coordinate intermediate. Stereoviews of the active sites of (A) wild-type LrPduO (PDB ID: 3CI1, St. Maurice et al., 2008) and (B) LrPduOF112A with the individual subunits of the trimer represented as ribbons colored in blue, red and brown. For clarity, only the active site at the interface of the red and blue subunits is shown. The carbon atoms of cob(II)alamin are colored in black while the carbon atoms of ATP are colored in green. The potassium (purple sphere) and magnesium (green sphere) atoms are also displayed. The electron density corresponding to the DMB portion of cob(II)alamin is disordered in the wild-type structure.