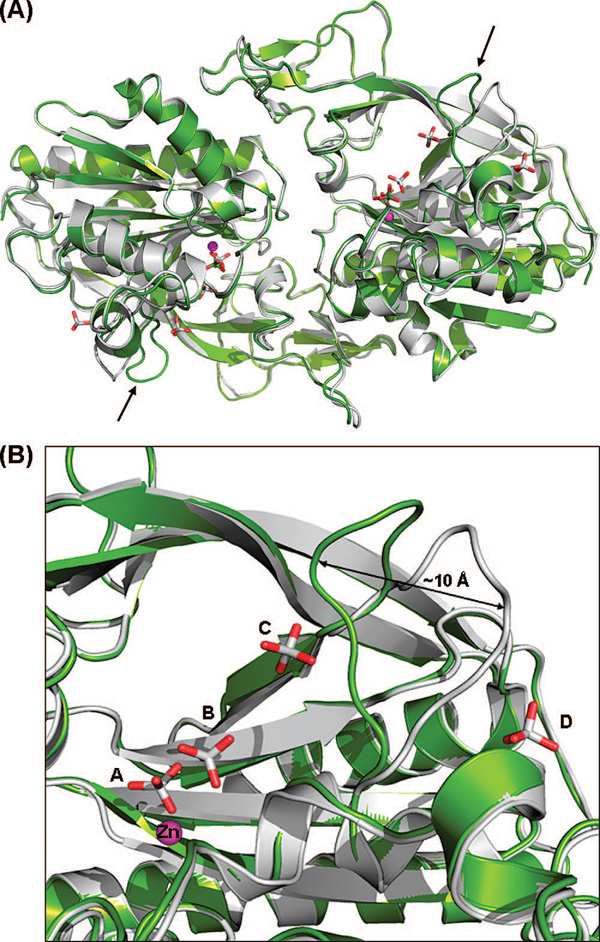
Figure 2.
(A) Comparison of the human apoenzyme structure of hACY2 (green) with the recently determined structure (PDB entry 2I3C) (10) of the same molecule (gray). The overlay was created by a least-squares superimposition of the Cα atoms of the complete polypeptide chain. The only major difference between the two structures is the position of the loop comprising residues 158–164, shown with black arrows. (B) Close-up view of the 158–164 loop in both structures. The difference in loop conformations between the two molecules is likely caused by the presence of additional phosphate ions (phosphates C and D) bound in the previously determined structure.