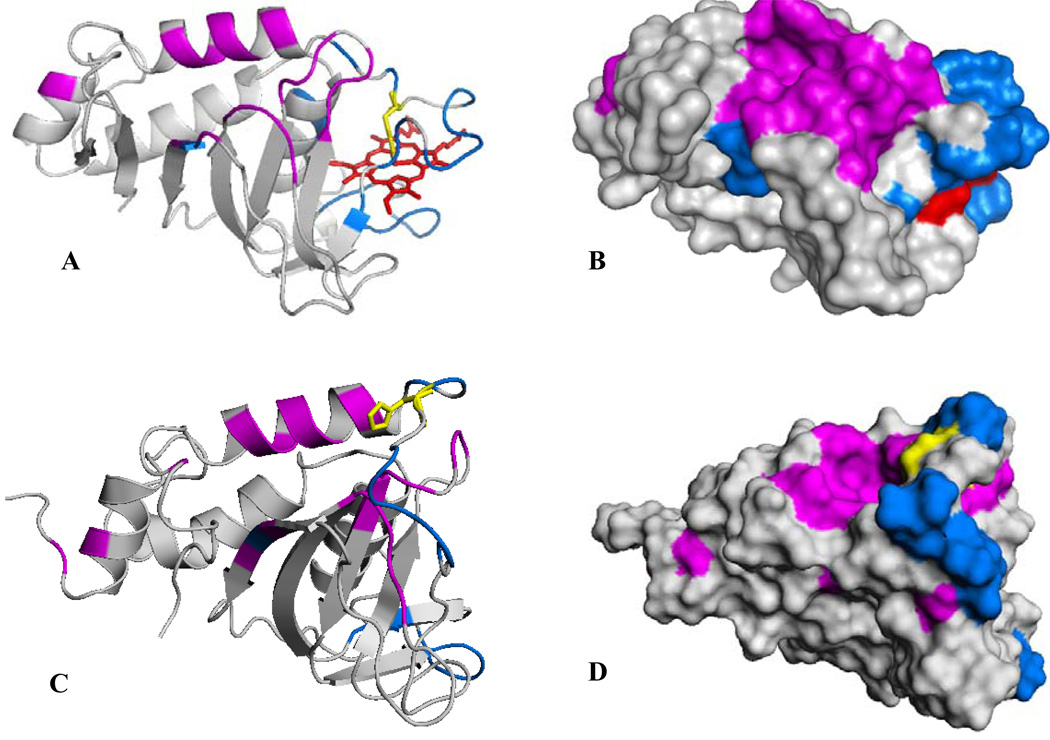
Figure 10.
(A) A view of the structure of truncated holo-HasAp where residues whose cross-peaks exhibit weighted chemical shift perturbations larger than three times the average of all chemical shifts (0.011) upon titration with met-Hb are highlighted in magenta and residues whose corresponding cross-peaks disappear upon addition of met-Hb are highlighted in blue. His32 is shown in yellow. (B) Surface representation of the view shown in (A). (C) A view of the structure of apo-HasAs (PDB ID 1YBJ) (45), where residues highlighted in magenta are equivalent to residues in holo-HasAp whose corresponding cross-peaks are affected by chemical shift perturbations and residues highlighted in blue are equivalent to those in holo- HasAp whose cross-peaks disappear upon titration with met-Hb. (D) Surface representation of the view shown in (C).