Figure 2.
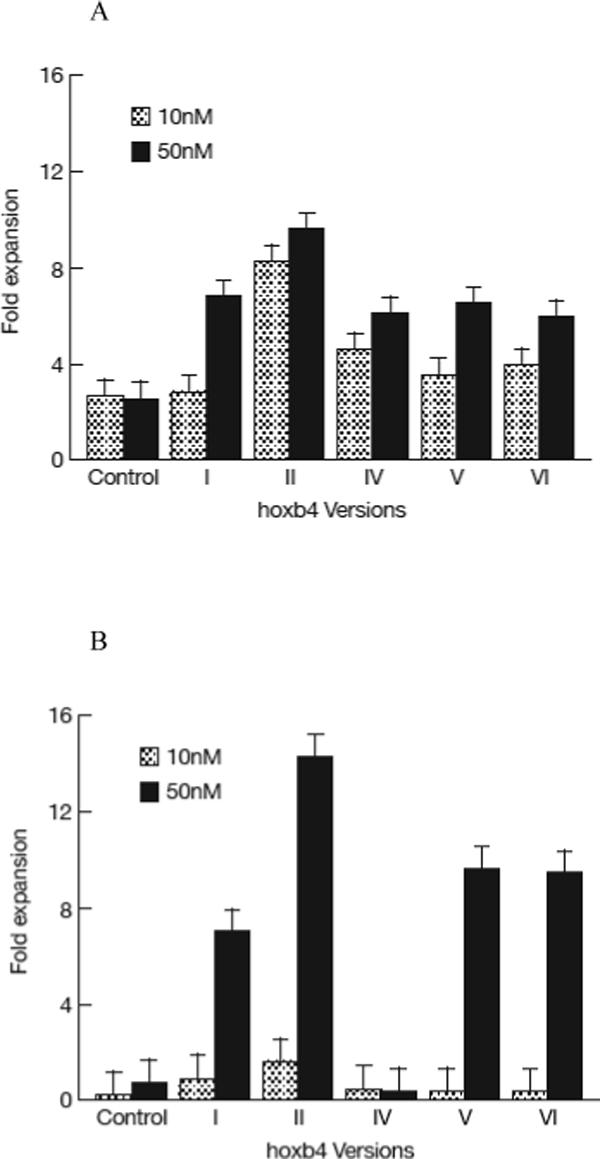
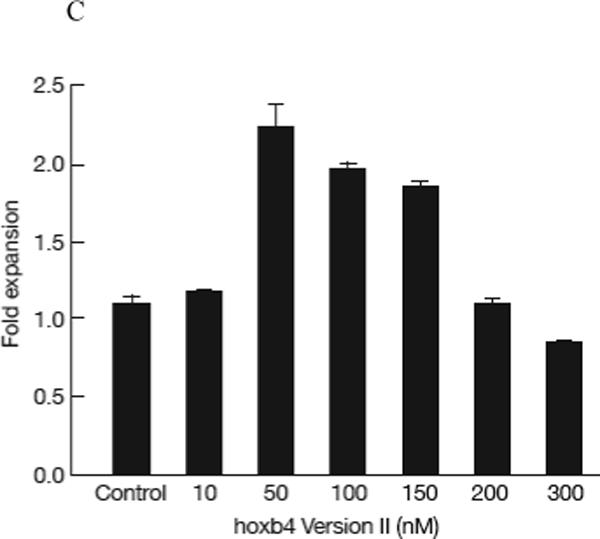
Expansion of HSCs by recombinant human hoxb4. 1−5 × 105 normal human BM cells were cultured in 1ml cytokine-supplemented StemSpan medium for 2 days, then recombinant hoxb4 proteins were added at desired concentrations once every 6 hours for 4 more days. Cells were harvested, 10% of the cells were used for CFC assay and 90% of the cells were used for LTC-IC assay. Overall, hoxb4 had a significant effect (P<0.01) in CFC expansion, of which version II at 10nM concentration and versions I, II, IV and V at 50nM concentrations significantly expanded CFC (P<0.05) compared with cultures without hoxb4 (A). Hoxb4 also had a significant effect (P<0.01) in expanding LTC-IC, of which all hoxb4 versions at 50nM concentration significantly expanded LTC-IC compared the controls (B). Among all five versions of hoxb4, version II had the highest activity in expanding fresh human BM cells. Dose response of version II hoxb4 (T-hoxb4-H) was tested in frozen human BM cells. T-hoxb4-H used at 50, 100, and 150nM concentrations showed significantly higher (P<0.05) CFC expansion than controls (C).
