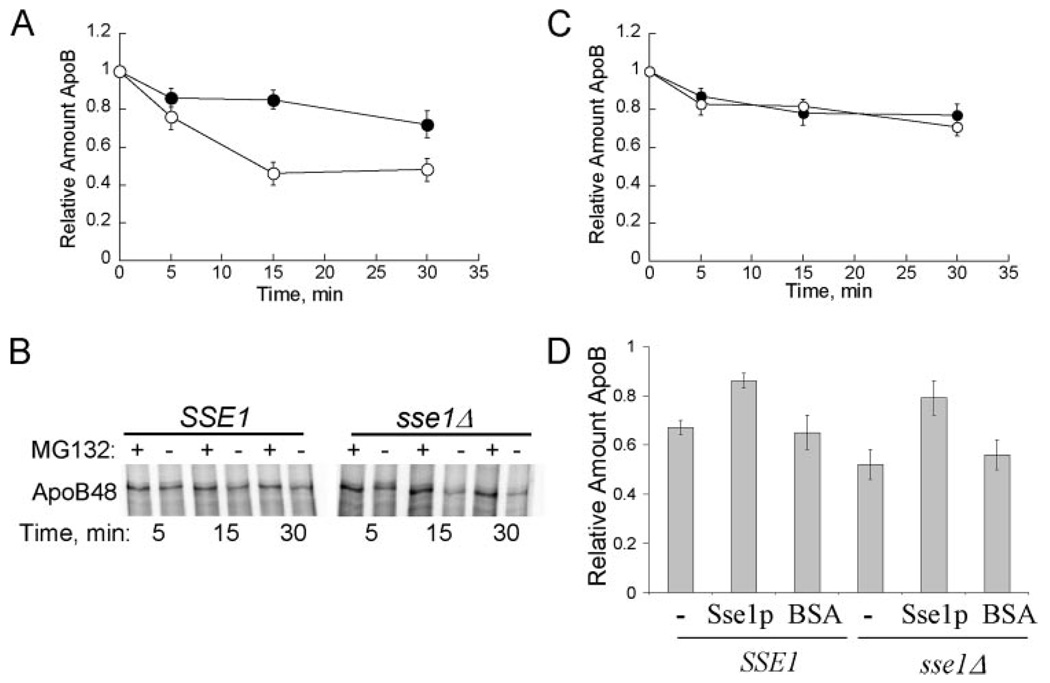
FIGURE 1. Sse1p contributes to apoB48 stabilization in vitro.
A, apoB48 degradation was assessed in vitro at 37 °C using cytosol (5 µg/µl) from SSE1 (●) or sse1Δ (JG014a) (○) yeast. The percentage of apoB remaining was calculated by normalizing the amount of apoB in the Me2SO-treated samples to those samples treated with MG132, thus reflecting only the degree of proteasome-mediated degradation. Data represent the means from six independent experiments ± S.E. of the means: 15 min, p < 0.0002; 30 min, p < 0.0005. B, phosphorimage of 35S-labeled apoB48 during a representative degradation assay in cytosol from SSE1 or sse1Δ (JG014a) yeast. Reactions were treated with the proteasome inhibitor MG132 (250 µm) or Me2SO (−) as indicated. C, apoB48 degradation is similar in cytosol from ssb1Δssb2Δ cells and the isogenic wild-type cells. ApoB48 degradation was assessed in vitro at 37 °C using cytosol (5 µg/µl) from SSB1SSB2 (●) or ssb1Δssb2Δ (○) yeast, and quantified as above. Data represent the means from six independent experiments ± S.E. of the means. D, degradation reactions containing the indicated source of cytosol at a final concentration of 5 µg/µl were supplemented with Sse1p at a final concentration of 3% of the total protein or an equal amount of bovine serum albumin (BSA). Reactions were incubated at 37 °C for 15 min. Data represent the means of 3–9 experiments ± S.E. of the mean. The activity of purified Sse1p was confirmed in steady state ATPase assays: 0.5 nmol of ATP hydrolyzed min−1 mg protein−1. SSE1, cytosol ± purified Sse1p, p < 0.02; sse1Δ, (JG014a) cytosol ± purified Sse1p, p < 0.002.