Abstract
eIF3f is a subunit of eIF3. We previously showed that eIF3f is phosphorylated by CDK11p46 which is an important effector in apoptosis. Here, we identified a second eIF3f phosphorylation site (Thr119) by CDK11p46 during apoptosis. We demonstrated that eIF3f is directly phosphorylated by CDK11p46 in vivo. Phosphorylation of eIF3f plays an important role in regulating its function in translation and apoptosis. Phosphorylation of eIF3f enhances the association of eIF3f with the core eIF3 subunits during apoptosis. Our data suggested that eIF3f may inhibit translation by increasing the binding to the eIF3 complex during apoptosis.
Keywords: eIF3f, cyclin-dependent kinase 11, translation initiation, phosphorylation, apoptosis, melanoma
1. Introduction
CDK11 is a member of the cyclin-dependent kinase family. Recent studies suggested that CDK11 interacts with cyclin L and may be involved in RNA processing or transcription in proliferating cells [1–3]. An important function of CDK11 is its contribution to apoptosis [4] [5]. Upon apoptotic stimulation, CDK11p110 is cleaved by caspases to generate a 46kDa isoform that contains the catalytic domain of CDK11p110 which can then phosphorylate other proteins [4,6,7].
We have identified eIF3f as a substrate of CDK11p46 [8]. eIF3 is the largest translation initiation factor that binds to 40S ribosomes and promotes the binding of methionyl-tRNA and mRNA [9,10]. eIF3f contains the MPN/Mov34 domain. We have demonstrated that CDK11p46 interacts with the Mov34 domain of eIF3f in vitro and in vivo, and the interaction can be strengthened by the stimulation of apoptosis [8]. eIF3f is phosphorylated by CDK11p46 at Ser46 during apoptosis. Furthermore, CDK11p46 inhibits translation both in vitro and in vivo. We also demonstrated that eIF3f is a negative regulator of translation [11]. Overexpression of eIF3f induces rRNA degradation and apoptosis [11]. Loss of the eIF3f gene allele has been observed in melanoma and pancreatic cancer [12,13]. These previous data provided insight into the function of CDK11p46 in apoptotic signaling and also suggest that eIF3f may be a downstream death executer of CDK11p46. This is accomplished by inhibiting overall cellular translation.
The biological consequence of the phosphorylation of eIF3f by CDK11p46 during apoptosis is not known. Here, we identified a second site in eIF3f that is phosphorylated by CDK11p46 during apoptosis. We showed that alteration of the phosphorylation status of eIF3f can significantly influence its function in the regulation of translation and apoptosis. We also demonstrated that the association between eIF3f and the core eIF3 complex is increased during apoptosis.
2. Materials and methods
2.1. Cell culture and treatments
A375 human melanoma cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured in RPMI 1640 medium supplemented with 5% FBS. Cells were transfected using Lipofectamine 2000 (Invitrogen).
2.2. Purification of recombinant protein from E. coli
GST, GST-eIF3f and its truncated proteins were purified and concentrated as described [8].
2.3. Kinase assay
The in vitro kinase assays were carried out as described [4] [8]. For in vivo kinase assay, two days after transfection, A375 cells were preincubated in phosphate-free medium for 2h. The medium was then replaced with medium containing 200 μCi of H332PO4 for 2.5h. The cells were then lysed and immunoprecipitated using eIF3f antibody. The incorporation of 32P was detected by autoradiography.
2.4. Identification of eIF3f phosphorylation site
Caspase-processed CDK11p46 was immunoprecipitated from staurosporine treated A375 cells. Recombinant eIF3f-1 protein was then phosphorylated by the CDK11p46 and analyzed for phosphoamino acids by MS as described [4] [8].
2.5. Immunoprecipitation and Western blotting
Immunoprecipitation and Western blotting was performed as described [8]. eIF3, eIF3f and CDK11 antibodies were raised in goat and rabbit as described [14] [8]. Phosphothreonine, α-tubulin, goat polyclonal eIF3a, eIF3η(eIF3b), and eIF3c antibodies were purchased from Sigma, Oncogene and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
2.6. 2D gel electrophoresis
The 2D gel electrophoresis was performed according to the ReadyStrip IPG strip instruction manual of Bio-Rad.
2.7. Quantitative real-time RT-PCR
Real-time RT-PCR was performed as described [8].
2.8. Cell free in vitro translation assay
A rabbit reticulocyte lysate in vitro translation system (Promega, Madison, WI) was used to measure the translation level, which is indicated by luciferase activity as previously described [8]. The recombinant proteins were purified as described previously by our group [8,11].
2.9. Apoptosis assay
Apoptosis was measured by Acridine Orange and Ethidium Bromide staining (100 μg/ml of each dye) and fluorescent microscopy as described [11]. Caspase 3/7 activity was measured using Caspase-Glo 3/7 Assay kit (Promega). Annexin V/PI staining and flow cytometry was also used to measure apoptosis.
2.10. Cell fractionation
A375 cells were washed with PBS and resuspended in hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5mM DTT) containing 1mM sodium orthovanadate, 1mM phenylmethylsulfonyl fluoride and 1% protease inhibitor cocktail (Sigma) and incubated on ice for 15 min. Cells were then homogenized and the percentage of nuclei was checked by trypan blue staining. Pellet was collected at 2000 g for 10 min. The supernatant contains the cytoplasm.
3. Results
3.1. CDK11p46 phosphorylates eIF3f at more than one site during apoptosis
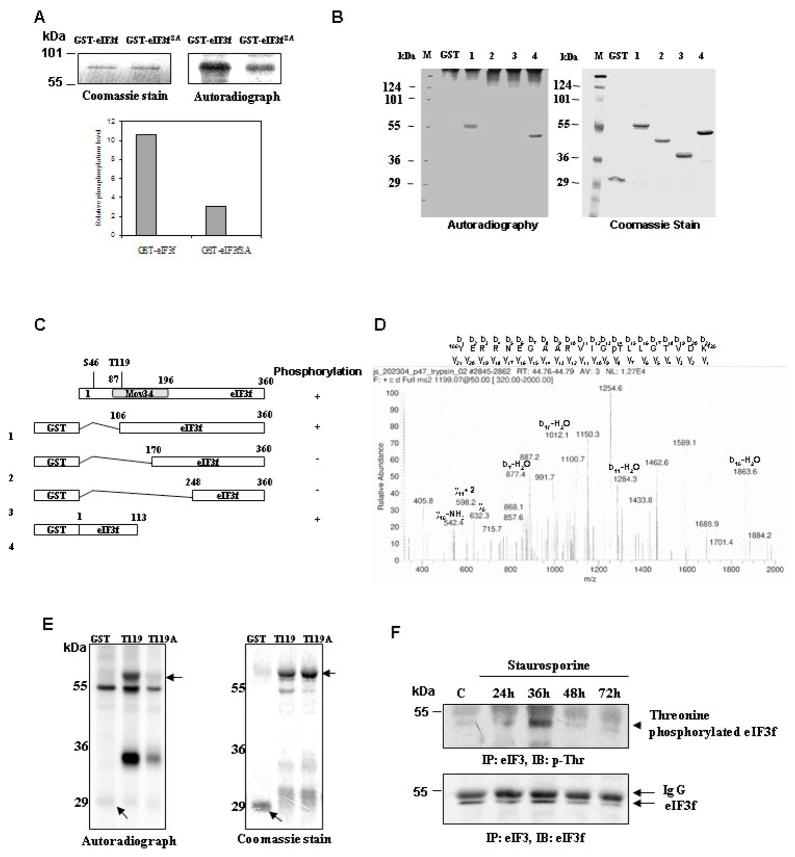
We have shown that CDK11p46 phosphorylates eIF3f at Ser46 [8]. To investigate if it is the only phosphorylation site in eIF3f that is phosphorylated by CDK11p46, Ser46 of eIF3f was mutated to alanine. A recombinant GST-eIF3fS46A mutant protein was made and incubated with CDK11p46 that immunoprecipitated from apoptotic cells. The phosphorylation level of eIF3fS46A by CDK11p46 was lower compared to wild type eIF3f, but there was still a significant level of phosphorylation (Fig. 1A). To investigate other phosphorylation sites, we used deletion constructs of eIF3f (Fig. 1C) as substrates in kinase assays. CDK11p46 was immunoprecipitated from apoptotic cell lysate and incubated with equal amount of the indicated substrates (Fig. 1B, right panel). Phosphorylation was seen with eIF3f deletions #1 and #4, but not with GST control, #2 and #3 (Fig. 1B, left panel). Ser46 is present in eIF3f-4 protein, but not in eIF3f-1 protein (Fig. 1C), and eIF3f-2 is not phosphorylated. These data suggested that additional phosphorylation sites might exist between residues 106 and 170 in eIF3f.
Fig. 1.
CDK11p46 phosphorylates eIF3f at more than one site during apoptosis. (A) Kinase assay: CDK11p46 was immunoprecipitated from apoptotic A375 cells and incubated with GST-eIF3f or eIF3fS46A. (B) Kinase assay was performed with GST or four truncated eIF3f proteins as substrates. (C) Diagram of Mov34 domain and four deletion constructs of eIF3f. (D) MS identification of Thr119 phosphorylation. (E) Kinase assays were performed with eIF3f-1 (T119) and eIF3f-1 T119A (T119A) as substrates. (F) Staurosporine treated A375 cells were immunoprecipitated with eIF3 antibody followed by immunoblot with phosphothreonine or eIF3f antibody.
To identify additional phosphorylation site(s) in eIF3f, the truncated eIF3f-1 protein was incubated with CDK11p46 immunoprecipitated from apoptotic cells. After the kinase reaction, eIF3f-1 was separated by SDS-PAGE, digested in-gel with trypsin and subjected to mass spectrometry (MS) analysis. The spectrum for the eIF3f-1 phosphopeptide (residues Y106 through K126) is shown in Fig. 1D. Identification of a phosphorylated Thr119 is seen by analysis of b ions and y ions. A gain of phosphate after the y8 or b14 ion was observed. To verify the MS result, we mutated Thr119 to alanine in eIF3f-1 to create eIF3f-1TA and used it as the substrate in a kinase assay. It is clear that the T119A mutation abolished the phosphorylation by CDK11p46 (Fig. 1E).
We then investigated the endogenous threonine phosphorylation status of eIF3f in apoptotic cells. The threonine phosphorylation of eIF3f was seen and the maximum phosphorylation level was seen at 36 h after treatment (Fig. 1F). This data is consistent with our previous in vitro kinase assay data and endogenous serine phosphorylation data that eIF3f is phosphorylated by CDK11p46 after apoptotic stimulation, especially around 36 h [8]. Hence, threonine phosphorylation of eIF3f, likely by CDK11p46, also occurs endogenously during apoptosis.
3.2. Endogenous phosphorylation of eIF3f by CDK11p46
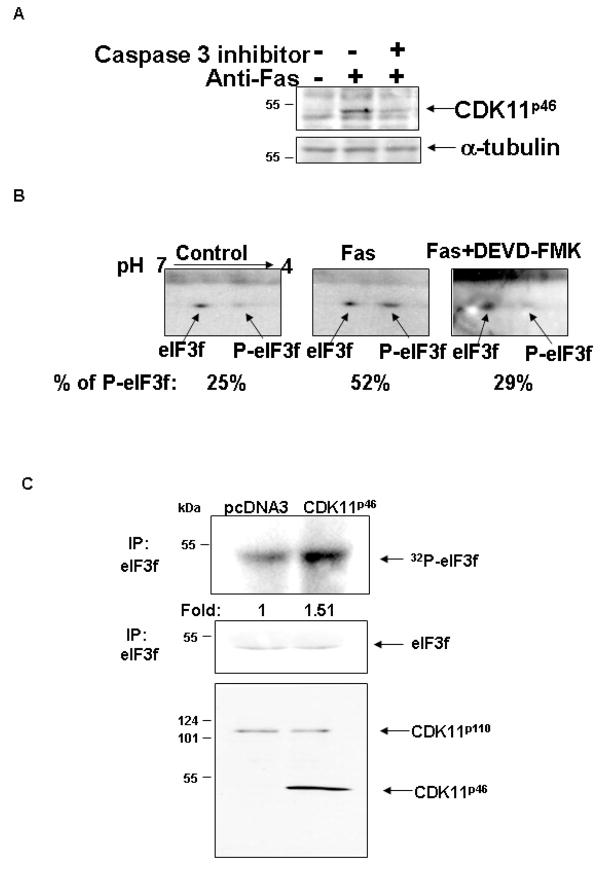
To further confirm the endogenous phosphorylation of eIF3f by CDK11p46 during apoptosis, A375 cells were treated with anti-Fas and/or caspase 3 inhibitor (DEVD-FMK). [CDK11p46 was produced in anti-Fas treated cells, whereas caspase 3 inhibitor blocked the production of CDK11p46 (Fig. 2A). eIF3f was immunoprecipitated from these cells followed by two dimensional SDS-PAGE and immunoblot with eIF3f antibody. Phosphorylated eIF3f was separated from un-phosphorylated eIF3f by different pI. Under normal condition, 25% of endogenous eIF3f protein is phosphorylated (Fig. 2B). During apoptosis, phosphorylated eIF3f is significantly increased to 52% of total eIF3f. The addition of caspase 3 inhibitor reversed this phosphorylation (Fig. 2B). This observation is consistent with our previous data supporting that eIF3f is phosphorylated endogenously during apoptosis and this is presumably done by CDK11p46.
Fig. 2.
Endogenous eIF3f is phosphorylated by CDK11p46 during apoptosis. A375 cells were treated with 0.5 μg/ml anti-Fas with or without pretreatment with caspase 3 inhibitor (DEVD-FMK) for 36 hr. (A) Cells were lysed and immunobloted with CDK11 and α-tubulin antibodies. (B) 2D- SDS-PAGE showed increased phosphorylation of eIF3f during apoptosis. (C) Increased phosphorylation of endogenous eIF3f in CDK11p46 transfected cells.
To analyze whether endogenous eIF3f is directly phosphorylated by CDK11p46, A375 cells were transfected with CDK11p46. Immunoprecipitated eIF3f has a 50% higher phosphorylation level in CDK11p46 transfected cells compared to vector transfected cells (Fig. 2C). The membrane was also blotted with eIF3f antibody to show that the higher phosphorylation level is not due to higher protein level of eIF3f. This result strongly suggests that eIF3f is directly phosphorylated by CDK11p46 in living cells.
3.3. Phosphorylation of eIF3f by CDK11p46 regulates its function in translation and apoptosis
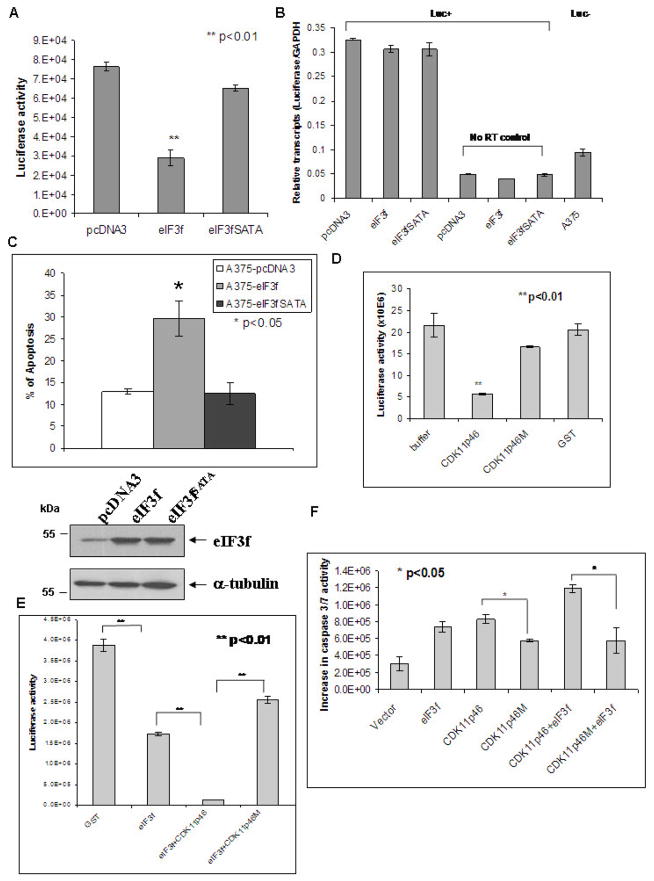
We have shown that eIF3f inhibits translation and induces apoptosis in tumor cells [11]. To examine whether phosphorylation of eIF3f by CDK11p46 regulates its function, we mutated the Ser46 and Thr119 to alanine (A). This yielded eIF3fSATA. Translation was compared between wild type and mutated eIF3f transfected cells using a luciferase reporter system. Luciferase synthesis inhibition was significantly attenuated in eIF3fSATA-transfected cells (Fig. 3A). This effect was not at the mRNA level as confirmed by real-time RT-PCR, but at the translation level (Fig. 3B). Alanine mutation also diminished the apoptosis induction by wild type eIF3f (Fig. 3C). These results indicated that the eIF3f phosphorylation regulates its function in translation and apoptosis.
Fig. 3.
Phosphorylation of eIF3f by CDK11p46 is important for the inhibition of translation and induction of apoptosis. (A) A375 cells were co-transfected with pcDNA3, or pcDNA3-eIF3f, or pcDNA3-eIF3fSATA and pGL3-SV40 carrying the luciferase reporter gene. At 24 h post-transfection, cells were lysed and luciferase activity was measured by a luminometer. (B) Real-time RT-PCR was performed as described. Untransfected A375 cells and reactions without reverse transcription served as controls. (C) Apoptosis was measured by Acridine Orange/Ethidium Bromide staining. (D) (E) Luciferase mRNA was translated in an in vitro translation system in the presence of buffer, GST, or indicated recombinant proteins. Synthesized luciferase activity was measured with a luminometer. (F) A375 cells were transfected with indicated plasmids. Caspase 3/7 activity was measured 48h after transfection.
To further examine if CDK11p46 is responsible for these effects, we used a kinase dead mutant form of CDK11p46 - CDK11p46M [8]. We have shown that CDK11p46M can not phosphorylate eIF3f [8]. In the in vitro translation assay, wild-type CDK11p46 significantly inhibited translation while CDK11p46M attenuated the inhibition (Fig. 3D). As shown previously by our group, eIF3f can inhibit translation (Fig. 3E) [11]. When eIF3f and CDK11p46 were added together, they inhibited translation synergetically (Fig. 3E). However, CDK11p46M abolished this synergetic effect (Fig. 3E). CDK11p46M also attenuated the apoptosis induction by CDK11p46 (Fig. 3F). These results further supported that CDK11p46 is the kinase that regulates eIF3f function.
3.4. Phosphorylation by CDK11p46 increases the association of eIF3f with the eIF3 core complex during apoptosis
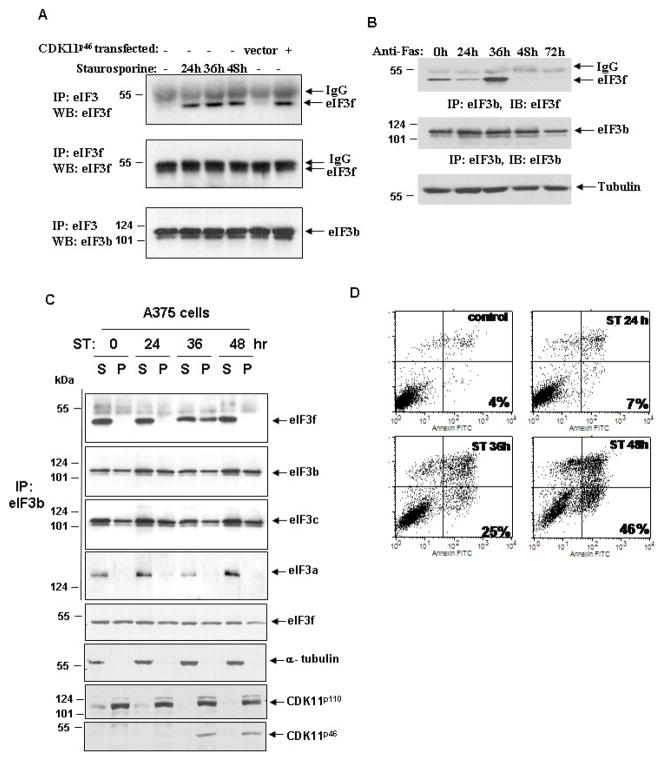
We next investigated the effect of eIF3f phosphorylation by CDK11p46 on its association with the eIF3 core complex during apoptosis. We found that there were equivalent amounts of total eIF3f in cells whether or not they underwent apoptosis (Fig. 4A, second panel). However, we saw a significant increase of endogenous eIF3f that associates with eIF3 complex by co-immunoprecipitation after apoptotic stimulation, especially at 36h (Fig. 4A, first panel). This increase is specific because another eIF3 subunit eIF3b remains the same during apoptosis (Fig. 4A, third panel). To verify our findings, we performed co-immunoprecipitation with specific antibodies against the eIF3b and eIF3f. Consistent with our previous observation, we observed an increased interaction of eIF3f with eIF3b at 36h after apoptotic stimulation (Fig. 4B, first panel). The association of eIF3f with eIF3b diminished after 48 h. There was no significant change in eIF3b protein level during apoptosis. These results indicated that endogenous eIF3f specifically increases its association with other eIF3 subunits during apoptosis.
Fig. 4.
Phosphorylation of eIF3f by CDK11p46 increases the association of eIF3f with other eIF3 subunits during apoptosis. A375 cells were treated with staurosporine or anti-Fas for indicated time. (A) Cell lysates were immunoprecipitated with eIF3 or eIF3f antibody followed by immunoblot with eIF3f or eIF3b antibody. The same experiment was carried out with CDK11p46 transfected cells. (B) Cell lysates were immunoprecipitated with eIF3b antibody followed by immunoblot with eIF3f or eIF3b antibody. (C) Cells were fractionated and immunoprecipitated with eIF3b antibody followed by immunoblot with eIF3f, eIF3b, eIF3c and eIF3a antibodies. Cell fractions were also immunoblotted with eIF3f, CDK11 and α-tubulin antibodies without immunoprecipitation. S: soluble fraction; P: pellet fraction. (D) Cells were analyzed for apoptosis using Annexin V/PI staining and flow cytometry analysis.
To further confirm that the increased association of eIF3f with the eIF3 core complex during apoptosis is related to the phosphorylation of eIF3f by CDK11p46, we performed a similar experiment using CDK11p46 transfected cells. We noted an increased association of eIF3f to the eIF3 complex only in CDK11p46 transfected cells, but not in vector control cells (Fig. 4A). This result further verified that CDK11p46 is responsible for the increased association of eIF3f with the eIF3 core complex.
To determine in which subcellular fraction there was increased eIF3f association with the eIF3 core complex during apoptosis, we performed cell fractionation in A375 cells. Most nuclei were in the pellet (P) fraction as was observed by a microscope and the location of CDK11p110 which is mainly a nuclear protein (Fig. 4C). Cytoplasm was mainly in the soluble (S) fraction, as proved by the location of α-tubulin which is a cytoplasmic protein (Fig. 4C). We found that endogenous eIF3f associates with eIF3b contiguously in the S fraction during apoptosis (Fig. 4C). However, eIF3f only associated with eIF3b in the P fraction at 36h after apoptotic stimulation. There was no change in the interaction between eIF3b and eIF3a or eIF3c during apoptosis (Fig. 4C). eIF3f and two of the core subunits, eIF3b and eIF3c, were localized in both S and P fractions during apoptosis, but more in the S fraction. eIF3a is predominantly localized in the S fraction.
We confirmed apoptosis induction by Annexin V staining and flow cytometry analysis. There was a gradual increase in apoptosis over time, up to 46% of apoptosis at 48h (Fig. 4D). CDK11p46 was produced 24h after apoptotic stimulation. It peaked at 36h and then started to decrease after 48h (Fig. 4C, last row). Furthermore, CDK11p46 was predominantly located in the P fraction where eIF3f increased its association with eIF3b. Therefore, the events of CDK11p46 production, eIF3f phosphorylation, and the association of eIF3f with eIF3b occur coincidentally with each other.
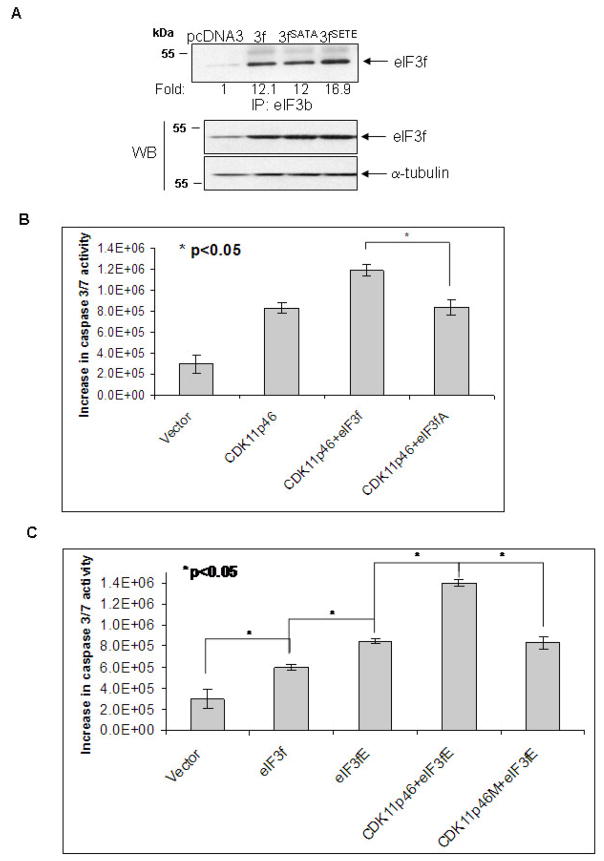
To investigate whether the specific eIF3f phosphorylation by CDK11p46 alters the association between eIF3f and eIF3 core complex, we performed co-immunoprecipitation with specific antibodies against the eIF3b and eIF3f in eIF3f, eIF3fSATA or eIF3fSETE (phosphorylation mimic mutant) transfected cells. As predicted, there is a 40% increased association between eIF3fSETE and eIF3b (Fig. 5A). The association between eIF3fSATA and eIF3b is only slightly less than eIF3f presumably due to the low level of endogenous phosphorylation of eIF3f in un-stimulated cells. To examine whether eIF3fSATA mutant attenuates apoptosis in CDK11p46 overexpressing cells, we co-transfected CDK11p46 with wild type or mutant eIF3f followed by apoptosis assay. As predicted, eIF3fSATA mutant did attenuate apoptosis in CDK11p46 overexpressing cells compared to wild type eIF3f (Fig. 5B). To investigate whether the phosphor-mimic eIF3fSETE mutant promotes apoptosis, we transfected eIF3fSETE alone or with CDK11p46 or CDK11p46M into A375 cells. eIF3fSETE mutant alone did induce more apoptosis than wild type eIF3f (Fig. 5C). Co-transfection with both eIF3fSETE and CDK11p46 induced even more apoptosis than eIF3fSETE alone, presumably due to the phosphorylation of the endogenous eIF3f (Fig. 5C). Furthermore, CDK11p46M mutant attenuated this synergetic effect (Fig. 5C). These results further indicated that the phosphorylation of eIF3f by CDK11p46 contributes to its increased association with eIF3 core complex and apoptosis.
Fig. 5.
The effect of the eIF3f phosphorylation sites mutation on its association with eIF3b and apoptosis. (A) A375 cells were transfected with pcDNA3, eIF3f, eIF3fSATA (phosphorylation sites inactivated) or eIF3fSETE (phosphorylation mimic) plasmids. Cell lysates were immunoprecipitated with eIF3b antibody followed by immunoblot with eIF3f antibody. Cell lysates were also used for Western blot using eIF3f and α-tubulin antibodies. (B) (C) A375 cells were transfected with indicated plasmids. Caspase 3/7 activity was measured 48h after transfection.
4. Discussion
We identified another phosphorylation site (Thr119) in eIF3f that is phosphorylated by CDK11p46 during apoptosis. Thr119 is located in the Mov34 domain of eIF3f which is important for both the translational inhibitory function of eIF3f [11] and the protein-protein interaction of eIF3f with CDK11p46[8]. It was also suggested by another group that the Mov34 domain plays an important role in complex assembly [15]. There is the possibility that phosphorylation of this site regulates the function of Mov34 domain. Further studies are necessary to clarify this issue. We have also presented data to show the direct phosphorylation of eIF3f by CDK11p46 endogenously which confirmed our previous observations. Results from the present study suggest that phosphorylation of eIF3f by CDK11 may contribute to the regulation of protein synthesis and apoptosis.
Our present study suggested that the association of eIF3f with the eIF3 complex appears to be strengthened in the insoluble fraction of cells during apoptosis. This observation suggested that during apoptosis, eIF3f was incorporated into a larger complex or organelle that also contains eIF3b and eIF3c, but not eIF3a. This larger complex and organelle can be precipitated with the nucleus in the insoluble fraction of the cell. eIF3f is also associated with a smaller complex in the soluble fraction of the cell consisting of eIF3a, b and c in both control and apoptotic cells. It was suggested that eIF3 is a dynamic complex. Our data also supports this hypothesis. eIF3 complexes in the cell may contain different subunits and variable numbers of different subunits. According to our present data, there are at least two different types of eIF3 complexes that exist in the cell. One eIF3 complex lacks eIF3a and eIF3f, while the other eIF3 complex consists of eIF3a-c and eIF3f. These different eIF3 complexes are localized in different subcellular fractions. Based on our observations, the model that we propose depicts that the phosphorylation of eIF3f by CDK11p46 enhances its binding to different sub-fractions of the eIF3 complex during apoptosis. This may regulate translation initiation.
A significant inhibition of overall protein synthesis has been observed in various cell types when cells are committed to apoptosis [18–20]. Presently it is not completely clear how the eIF3f phosphorylation regulates protein synthesis and apoptosis. However, we have shown previously that overexpression of eIF3f induces the degradation of 28S rRNA and decreases the 60S ribosomal subunit [11]. There is a physiologic link between rRNA degradation and the inhibition of protein synthesis during apoptosis. 28S rRNA is selectively degraded in Jurkat T cells and U937 cells by death receptor engagement [16]. It was suggested that the ribosome is a specific target for death effectors during apoptosis and that a caspase/Bcl2 independent pathway exists to activate its destruction. Therefore, phosphorylation of eIF3f may regulate its role in ribosome degradation, which may contribute to apoptosis. Our data also suggested that the phosphorylated eIF3f may predominantly localize to the nucleus. Other eIF3 subunits, such as eIF3a, eIF3e and eIF3b, have been reported to have nuclear localization and Dunand-Sauthier et al suggested that eIF3 is a dynamic complex [23–25]. Our data also suggest that eIF3 complex may exist in both cytoplasm and nucleus. The cytoplasmic eIF3 complex contains all of the eIF3 subunits, whereas the nuclear eIF3 complex contains only some eIF3 subunits. According to our present data, the nuclear eIF3 complex at least contains eIF3b and eIF3c, with phosphorylated eIF3f joining the complex during apoptosis. However, the nuclear eIF3 complex is highly possible to have functions other than translation initiation. It is known that the synthesis of ribosomes in eukaryotes takes place in nucleus [26] and then ribosomes were exported to the cytoplasm. There was evidence that eIF3j has a dual function in processing 20S pre-rRNA and translation initiation [27]. Cyclin-dependent kinases (CDKs) have been suggested to be involved in ribosome biogenesis and nucleolar organization [28]. Therefore, CDK11p46 may regulate the function of the nuclear eIF3 complex by phosphorylating eIF3f. Further studies of how eIF3f phosphorylation regulates its function will refine insights into the mechanism and regulation of translation initiation, apoptotic signaling, and tumorigenesis.
Acknowledgments
We thank Dr. George Tsaprailis of the SWEHSC/AZCC proteomics core at the University of Arizona for performing the MS. We also thank the flow cytometry core at the University of Arizona for the apoptosis assay. This work was supported by Cancer Biology Training Grant CA 09213, Faculty Small Grant Program (University of Arizona), ABRC grant (0007), grants CA133449 (JS), GI SPORE CA095060 (JS), CA70145 (MN), GM22135 (JH), Center grants ES066694, and NIH Arizona Cancer Center Support Grant CA023074.
Abbreviations
- CDK11
cyclin dependent kinase 11
- Ct
threshold cycle
- eIF3
eukaryotic initiation factor 3
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- GST
glutathione S-transferase
- MS
mass spectrometry
- m/z
mass to charge ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loyer P, Trembley JH, Lahti JM, Kidd VJ. The RNP protein, RNPS1, associates with specific isoforms of the p34cdc2-related PITSLRE protein kinase in vivo. J Cell Sci. 1998;111 (Pt 11):1495–1506. doi: 10.1242/jcs.111.11.1495. [DOI] [PubMed] [Google Scholar]
- 2.Trembley JH, Hu D, Hsu LC, Yeung CY, Slaughter C, Lahti JM, Kidd VJ. PITSLRE p110 protein kinases associate with transcription complexes and affect their activity. J Biol Chem. 2002;277:2589–2596. doi: 10.1074/jbc.M109755200. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson LA, Edgar AJ, Ehley J, Gottesfeld JM. Cyclin L is an RS domain protein involved in pre-mRNA splicing. J Biol Chem. 2002;277:25465–25473. doi: 10.1074/jbc.M202266200. [DOI] [PubMed] [Google Scholar]
- 4.Ariza ME, Broome-Powell M, Lahti JM, Kidd VJ, Nelson MA. Fas-induced apoptosis in human malignant melanoma cell lines is associated with the activation of the p34(cdc2)-related PITSLRE protein kinases. J Biol Chem. 1999;274:28505–28513. doi: 10.1074/jbc.274.40.28505. [DOI] [PubMed] [Google Scholar]
- 5.Shi L, Nishioka WK, Th’ng J, Bradbury EM, Litchfield DW, Greenberg AH. Premature p34cdc2 activation required for apoptosis. Science. 1994;263:1143–1145. doi: 10.1126/science.8108732. [DOI] [PubMed] [Google Scholar]
- 6.Beyaert R, et al. Cleavage of PITSLRE kinases by ICE/CASP-1 and CPP32/CASP-3 during apoptosis induced by tumor necrosis factor. J Biol Chem. 1997;272:11694–11697. doi: 10.1074/jbc.272.18.11694. [DOI] [PubMed] [Google Scholar]
- 7.Tang D, Gururajan R, Kidd VJ. Phosphorylation of PITSLRE p110 isoforms accompanies their processing by caspases during Fas-mediated cell death. J Biol Chem. 1998;273:16601–16607. doi: 10.1074/jbc.273.26.16601. [DOI] [PubMed] [Google Scholar]
- 8.Shi J, Feng Y, Goulet AC, Vaillancourt RR, Sachs NA, Hershey JW, Nelson MA. The p34cdc2-related Cyclin-dependent kinase 11 Interacts with the p47 Subunit of Eukaryotic Initiation Factor 3 during Apoptosis. J Biol Chem. 2003;278:5062–5071. doi: 10.1074/jbc.M206427200. [DOI] [PubMed] [Google Scholar]
- 9.Clemens MJ, Bushell M, Jeffrey IW, Pain VM, Morley SJ. Translation initiation factor modifications and the regulation of protein synthesis in apoptotic cells. Cell Death Differ. 2000;7:603–615. doi: 10.1038/sj.cdd.4400695. [DOI] [PubMed] [Google Scholar]
- 10.Sonenberg N, Hershey JWB, Mathews M. Cold Spring Harbor monograph series. Vol. 39. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. pp. xpp. 1020pp. 33–88. [Google Scholar]
- 11.Shi J, Kahle A, Hershey JW, Honchak BM, Warneke JA, Leong SP, Nelson MA. Decreased expression of eukaryotic initiation factor 3f deregulates translation and apoptosis in tumor cells. Oncogene. 2006;25:4923–4936. doi: 10.1038/sj.onc.1209495. [DOI] [PubMed] [Google Scholar]
- 12.Doldan A, Chandramouli A, Shanas R, Bhattacharyya A, Cunningham JT, Nelson MA, Shi J. Loss of the eukaryotic initiation factor 3f in pancreatic cancer. Mol Carcinog. 2008;47:235–244. doi: 10.1002/mc.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doldan A, Chandramouli A, Shanas R, Bhattacharyya A, Leong SP, Nelson MA, Shi J. Loss of the eukaryotic initiation factor 3f in melanoma. Mol Carcinog. 2008 Mar 31; doi: 10.1002/mc.20436. (Advanced online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer LJ, Milburn SC, Hershey JW. Immunochemical characterization of mammalian protein synthesis initiation factors. Biochemistry. 1982;21:4206–4212. doi: 10.1021/bi00261a003. [DOI] [PubMed] [Google Scholar]
- 15.Hoareau Alves K, Bochard V, Rety S, Jalinot P. Association of the mammalian proto-oncoprotein Int-6 with the three protein complexes eIF3, COP9 signalosome and 26S proteasome. FEBS Lett. 2002;527:15–21. doi: 10.1016/s0014-5793(02)03147-2. [DOI] [PubMed] [Google Scholar]
- 16.Nadano D, Sato TA. Caspase-3-dependent and -independent degradation of 28 S ribosomal RNA may be involved in the inhibition of protein synthesis during apoptosis initiated by death receptor engagement. J Biol Chem. 2000;275:13967–13973. doi: 10.1074/jbc.275.18.13967. [DOI] [PubMed] [Google Scholar]
- 17.King KL, Jewell CM, Bortner CD, Cidlowski JA. 28S ribosome degradation in lymphoid cell apoptosis: evidence for caspase and Bcl-2-dependent and -independent pathways. Cell Death Differ. 2000;7:994–1001. doi: 10.1038/sj.cdd.4400731. [DOI] [PubMed] [Google Scholar]
- 18.Morley SJ, McKendrick L, Bushell M. Cleavage of translation initiation factor 4G (eIF4G) during anti-Fas IgM-induced apoptosis does not require signalling through the p38 mitogen-activated protein (MAP) kinase. FEBS Lett. 1998;438:41–48. doi: 10.1016/s0014-5793(98)01269-1. [DOI] [PubMed] [Google Scholar]
- 19.Clemens MJ, Bushell M, Morley SJ. Degradation of eukaryotic polypeptide chain initiation factor (eIF) 4G in response to induction of apoptosis in human lymphoma cell lines. Oncogene. 1998;17:2921–2931. doi: 10.1038/sj.onc.1202227. [DOI] [PubMed] [Google Scholar]
- 20.Wallach D. Cell death induction by TNF: a matter of self control. Trends Biochem Sci. 1997;22:107–109. doi: 10.1016/s0968-0004(97)01015-3. [DOI] [PubMed] [Google Scholar]
- 21.Sheikh MS, Fornace AJ., Jr Regulation of translation initiation following stress. Oncogene. 1999;18:6121–6128. doi: 10.1038/sj.onc.1203131. [DOI] [PubMed] [Google Scholar]
- 22.Bushell M, Wood W, Clemens MJ, Morley SJ. Changes in integrity and association of eukaryotic protein synthesis initiation factors during apoptosis. Eur J Biochem. 2000;267:1083–1091. doi: 10.1046/j.1432-1327.2000.01101.x. [DOI] [PubMed] [Google Scholar]
- 23.Chudinova EM, Ivanov PA, Nadezhdina ES. Large subunit of translation initiation factor--3 p170 contains potentially functional nuclear localization signals. Mol Biol (Mosk) 2004;38:684–691. [PubMed] [Google Scholar]
- 24.Watkins SJ, Norbury CJ. Cell cycle-related variation in subcellular localization of eIF3e/INT6 in human fibroblasts. Cell Prolif. 2004;37:149–160. doi: 10.1111/j.1365-2184.2004.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunand-Sauthier I, Walker C, Wilkinson C, Gordon C, Crane R, Norbury C, Humphrey T. Sum1, a component of the fission yeast eIF3 translation initiation complex, is rapidly relocalized during environmental stress and interacts with components of the 26S proteasome. Mol Biol Cell. 2002;13:1626–1640. doi: 10.1091/mbc.01-06-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granneman S, Baserga SJ. Ribosome biogenesis: of knobs and RNA processing. Exp Cell Res. 2004;296:43–50. doi: 10.1016/j.yexcr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Valasek L, Hasek J, Nielsen KH, Hinnebusch AG. Dual function of eIF3j/Hcr1p in processing 20 S pre-rRNA and translation initiation. J Biol Chem. 2001;276:43351–43360. doi: 10.1074/jbc.M106887200. [DOI] [PubMed] [Google Scholar]
- 28.Sirri V, Hernandez-Verdun D, Roussel P. Cyclin-dependent kinases govern formation and maintenance of the nucleolus. J Cell Biol. 2002;156:969–981. doi: 10.1083/jcb.200201024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen F, Wang M, O’Connor JP, He M, Tripathi T, Harrison LE. Phosphorylation of PPARgamma via active ERK1/2 leads to its physical association with p65 and inhibition of NF-kappabeta. J Cell Biochem. 2003;90:732–744. doi: 10.1002/jcb.10668. [DOI] [PubMed] [Google Scholar]