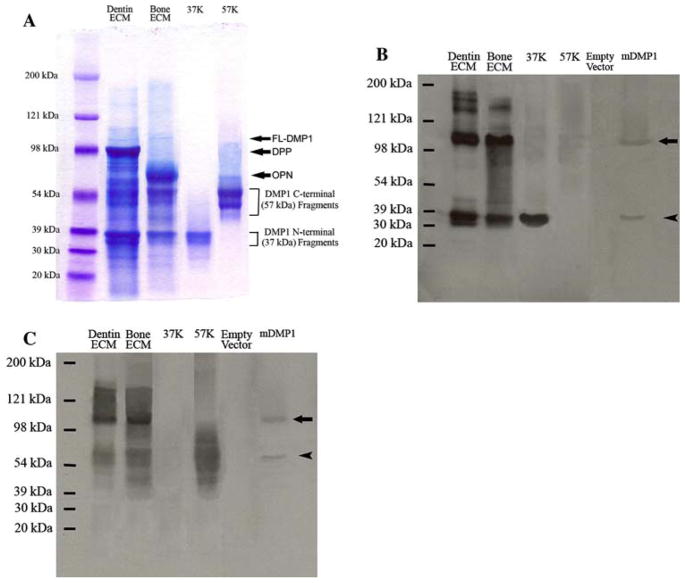
Fig. 2.
Detection of the full-length form of DMP1 and its processed fragments in the ECM extracts of rat dentin and bone. (A) Stains-All staining for Q-Sepharose chromatographic fractions of the ECM extracts of dentin and bone. Dentin ECM, The sample was from a chromatographic fraction (fraction 63, vertical arrow in Fig. 1A) of the Gdm-HCl/EDTA extract of dentin ECM. Sixty microliters of sample in 6 M urea solution was loaded. The thin, blue band migrating between 98 and 121 kDa represents the full-length form of DMP1 (FL-DMP1, estimated to be ~105 kDa). In this chromatographic fraction, the DMP1 fragments co-elute with the full-length form of DMP1 as well as DPP (approximate migrating position of DPP marked by arrow). Note that the DMP1 fragments are much more abundant than the full-length form in the dentin ECM. Bone ECM, The sample was from a chromatographic fraction (fraction 63, vertical arrow in Fig. 1B) of the Gdm-HCl/EDTA extract of rat bone ECM. Sixty microliters of sample in 6 M urea solution was loaded. The approximate migrating position of osteopontin (OPN) is indicated by arrow. 37K, Four micrograms of pure 37-kDa (NH2-terminal) fragment isolated from rat bone. 57K, Six micrograms of pure 57-kDa (COOH-terminal) fragment isolated from rat bone. Please note that both the 37- and 57-kDa fragments are present as clusters of bands, not a single protein band. The identification of these two clusters of bands as the NH2-terminal and COOH-terminal fragments of DMP1 was confirmed previously [12]. (B) Western immunoblotting using monoclonal anti-DMP1-N-9B6.3 antibody. Samples for the lanes of dentin ECM, bone ECM, 37K, and 57K are the same as in (A). Empty vector, Protein extract from cell lysates of HEK-293 cells transfected with a pcDNA3.1 vector that does not carry DMP1 cDNA; the total protein extract from one well of the HEK-293 cells of a six-well culture plate was loaded. mDMP1, protein extract from the cell lysates of the HEK-293 cells transfected with the mouse DMP1-DNA3.1 construct; the total protein extract from one well of the 293 cells of a six-well culture plate was loaded. Note the size of the recombinant full-length mouse DMP1 (~105 kDa, arrow) is identical to that of the full-length DMP1 detected in the ECM of rat dentin and bone. This monoclonal antibody recognizes both the full-length form (~105 kDa) and the NH2-terminal fragment (arrowhead) of DMP1. Note that the cleavage pattern in HEK-293 cells (a single cleavage) appears different from that occurring in the dentin and bone. (C) Western immunoblotting using polyclonal anti-DMP1-C-785 antibody. Loaded samples are the same as in (B). This polyclonal antibody recognizes both the full-length form (arrow) and the COOH-terminal fragment (arrowhead) of DMP1