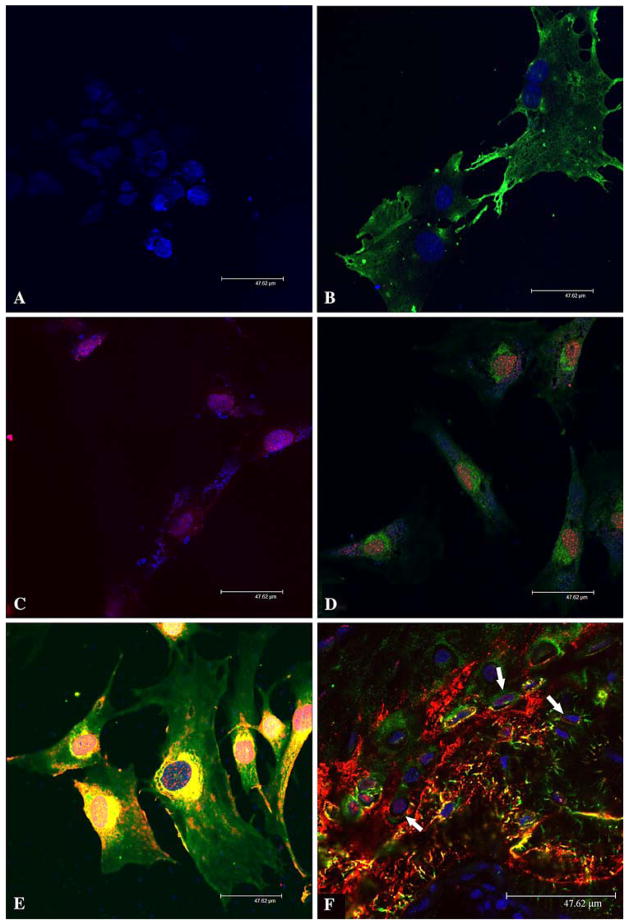
Fig. 4.
Immunofluorescence analysis of DMP1 in MC3T3-E1 cells and rat bone. Nuclei were stained by TO-PRO-3 fluorescent dye (blue) in all figures. Fluorescent immunostaining was assessed under a Leica SP2 scanning laser confocal microscope. Bar = 47.62 μm. (A) Immunofluorescence staining of nontransfected MC3T3-E1 cells (negative control). The preimmune serum was used in place of the primary anti-DMP1 antibodies. The secondary antibodies were a mixture of the goat anti-rabbit F(ab′)2 fragment conjugated with Alexa 488 (for green color) and goat anti-mouse F(ab′)2 fragment conjugated with Alexa 546 (for red color). Note that only the nuclei show a positive fluorescent signal (TO-PRO-3 staining, blue). (B) Immunofluorescence staining of nontransfected MC3T3-E1 cells. The primary antibody was the anti-DMP1-N-859 polyclonal antibody (made in rabbit). The secondary antibody was the goat anti-rabbit F(ab′)2 fragment conjugated with Alexa 488 (for green color). The signal for the NH2-terminal fragment of DMP1 (green) is observed in the cytoplasm but not in the nuclei. (C) Immunofluorescence staining of nontransfected MC3T3-E1 cells. The primary antibody was anti-DMP1-C-8G10.3 monoclonal antibody (made in mouse). The secondary antibody was the goat anti-mouse F(ab′)2 fragment conjugated with Alexa 546 (for red color). The signal for the COOH-terminal fragment (red) is observed in the nuclei. (D) Immunofluorescence staining of nontransfected MC3T3-E1 cells. The primary antibodies were a mixture of the anti-DMP1-N-859 polyclonal antibody and anti-DMP1-C-8G10.3 monoclonal antibody. The secondary antibodies were a mixture of the goat anti-rabbit F(ab′)2 fragment conjugated with Alexa 488 and goat anti-mouse F(ab′)2 fragment conjugated with Alexa 546. The signal for the NH2-terminal fragment of DMP1 (green color) is observed primarily in the cytoplasm, whereas that for the COOH-terminal fragment (red color) is mainly detected in the nuclei. (E) Immunofluorescence staining of MC3T3-E1 cells transfected with the DMP1-pcDNA3.1 construct. The primary and secondary antibodies are the same as in (D). Note the distribution difference between DMP1 fragments. (F) Immunofluorescence staining of osteocytes. The tissue section was from the ossified metaphysis region of the humerus of a 5-week-old rat. The primary and secondary antibodies were the same as in (D) and (E). Note that within the osteocytes (arrows) some signal for the COOH-terminal fragment of DMP1 (red) is observed in the nuclei. Also note that the NH2-terminal fragment is mainly found in the cytoplasm. In the cytoplasm, the NH2-terminal fragment of DMP1 appears more abundant along the processes of osteocytes