Fig. 3.
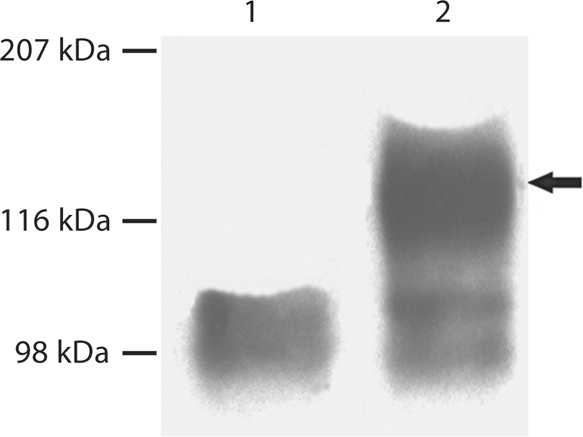
Substitution of Ser89 in mouse DMP1 completely prevented GAG glycosylation of DMP1. 300 μl of cell-conditioned culture medium was loaded onto SDS-PAGE, and the proteins were visualized by Western immunoblotting using a monoclonal antibody against the NH2-terminal region of DMP1 (anti-DMP1-N-clone 9B6.3). This monoclonal antibody [Qin et al., 2006] shows a strong reaction to DMP1-PG, but a weak reaction to the 37-kDa form. Lane 1: conditioned culture medium from cells transfected with the pcDNA3.1 construct carrying a mutant cDNA that encodes mouse DMP1, in which the GAG attachment residue, Ser89, was substituted by Gly89 (S89G). Lane 2: conditioned culture medium from cells transfected with the pcDNA3.1 construct carrying normal mouse DMP1 cDNA. Note the absence of DMP1-PG in lane 1 compared to the intact proteoglycan in lane 2 (arrow).
