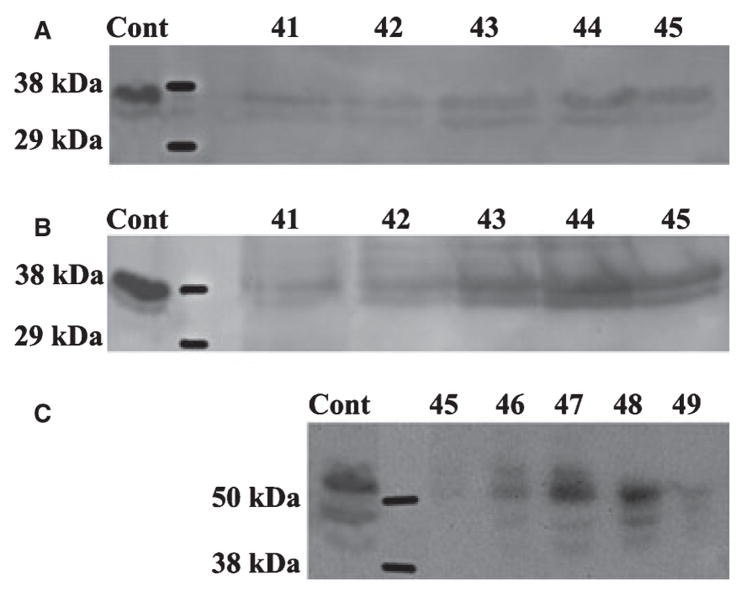
Fig. 3.
Western immunoblotting of dentin extracts using anti-DMP1 IgG2b. (A) The 37 kDa fragment of dentin matrix protein 1 (DMP1) was detected in fractions 41–45 of the dentin G1 extract by monoclonal anti-DMP1 IgG2b 9B6.3 that specifically recognizes the NH2-terminal region of DMP1. (B) The 37 kDa fragment of DMP1 was also detected in similar fractions of dentin E extract by antibody 9B6.3. (C) The 57 kDa fragment of DMP1 was detected in fractions 45–49 of dentin E extract by polyclonal anti-DMP1-C that specifically recognizes the COOH-terminal region of DMP1. Note that more than one immunoreactive band for anti-DMP1 was present. We believe that the double or triple bands of DMP1 represent the processed products of DMP1 resulting from cleavage at several sites (20). The positive control (Cont) in panels A and B was 4 μg of 37 kDa fragment purified from rat bone. The positive control (Cont) in panel C was 4 μg of 57 kDa fragment purified from rat bone.