Abstract
The best understood “fight or flight” mechanism for increasing heart rate (HR) involves activation of a cyclic nucleotide-gated ion channel (HCN4) by β-adrenergic receptor (βAR) agonist stimulation. HCN4 conducts an inward “pacemaker” current (If) that increases the sinoatrial nodal (SAN) cell membrane diastolic depolarization rate (DDR), leading to faster SAN action potential generation. Surprisingly, HCN4 knockout mice were recently shown to retain physiological HR increases with isoproterenol (ISO), suggesting that other If-independent pathways are critical to SAN fight or flight responses. The multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII) is a downstream signal in the βAR pathway that activates Ca2+ homeostatic proteins in ventricular myocardium. Mice with genetic, myocardial and SAN cell CaMKII inhibition have significantly slower HRs than controls during stress, leading us to hypothesize that CaMKII actions on SAN Ca2+ homeostasis are critical for βAR agonist responses in SAN. Here we show that CaMKII mediates ISO HR increases by targeting SAN cell Ca2+ homeostasis. CaMKII inhibition prevents ISO effects on SAN Ca2+ uptake and release from intracellular sarcoplasmic reticulum (SR) stores that are necessary for increasing DDR. CaMKII inhibition has no effect on the ISO response in SAN cells when SR Ca2+ release is disabled and CaMKII inhibition is only effective at slowing HRs during βAR stimulation. These studies show the tightly coupled, but previously unanticipated, relationship of CaMKII to the βAR pathway in fight or flight physiology and establish CaMKII as a critical signaling molecule for physiological HR responses to catecholamines.
Keywords: sarcoplasmic reticulum, cardiac pacemaker, calcium, isoproterenol, HCN4
Increased heart rate (HR) is a fundamental physiological component of the “fight or flight” response to β-adrenergic receptor (βAR) stimulation. Despite the critical importance of sinoatrial nodal (SAN) cardiac pacemaker cells to vertebrate physiology, the signaling mechanisms underlying βAR actions in SAN cells are not completely understood. The leading mechanistic paradigm for understanding HR increases during βAR stimulation is that increased 3′,5′ cyclic adenosine monophosphate (cAMP) increases “pacemaker” current (If) in SAN cells. However, surprising recent findings show that mice lacking the predominant If channel gene (HCN4) exhibit physiological HR responses to isoproterenol (ISO), despite a loss of ISO responsive If (1). The unexpected dispensability of HCN4 for SAN fight or flight responses led us to consider alternative models of SAN response to βAR stimulation.
Intracellular Ca2+ (Cai2+) release from sarcoplasmic reticulum (SR) stores induces myofilament crossbridge formation and contraction in ventricular myocytes, but SAN cells that lack a mechanical purpose also contain SR Ca2+ proteins (2). Cai2+ release from the SR may contribute to an If-independent pathway for increasing HR during βAR stimulation (3), suggesting the possibility that SAN cells use SR Ca2+ release machinery to regulate HR. The multifunctional Ca2+ and calmodulin-dependent protein kinase (CaMKII) is a serine-threonine kinase that is activated during ISO stimulation in ventricular myocardium (4). In ventricular myocytes, CaMKII activity is important for increasing SR Ca2+ filling and release (5) during ISO stimulation, but the potential role of CaMKII for regulating fight or flight SAN responses or SAN SR Ca2+ is unexplored. Given the possible importance of cellular Ca2+ in SAN function, we hypothesized that CaMKII activity is necessary for physiological HR responses to βAR stimulation.
Here, we show that CaMKII plays a previously unanticipated but decisive role to increase SAN rates during βAR stimulation. Studies in hearts from mice with SAN cell CaMKII inhibition suggest that CaMKII activity is required for chronotropic responses to ISO. CaMKII is selectively engaged in SAN cells during βAR stimulation and leads to coordinated enhancement of SR Ca2+ filling, greater diastolic SR Ca2+ release, and an increased diastolic depolarization rate (DDR) to increase HRs, independent of If. In contrast, CaMKII inhibition does not slow HRs or SAN cell action potential (AP) frequency in the absence of βAR stimulation or when SR Ca2+ release is disabled. These studies define a novel, CaMKII-dependent cellular mechanism for SAN fight or flight physiology.
Results
CaMKII Inhibition Reduces Fight or Flight HR Responses.
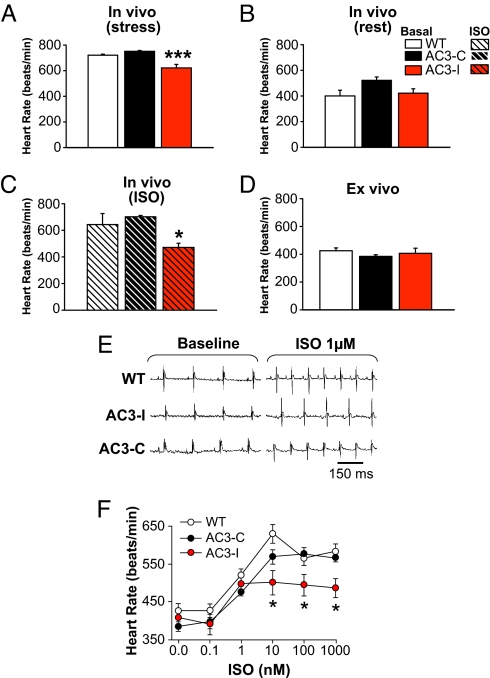
AC3-I is a peptide that mimics the CaMKII regulatory domain and acts as a highly specific CaMKII inhibitor (6). We developed transgenic mice with cardiomyocyte-specific AC3-I expression by placing a minigene encoding AC3-I under control of the α-myosin heavy chain promoter. AC3-I mice under stress imposed by physical restraint, necessary for the purpose of echocardiography, unexpectedly showed significantly slower HRs compared with wild-type (WT) or control mice with transgenic expression of an inactive, scrambled form of AC3-I, (AC3-C) (Fig. 1A) (4). HRs from resting, unrestrained mice with implanted ECG telemeters were lower than for stressed mice and similar between AC3-I and control mice (Fig. 1B). Plasma catecholamines were not significantly different between AC3-I and control mice, suggesting the slower HRs in stressed AC3-I mice were not due to lower catecholamine levels (Fig. S1A). ISO injection significantly increased HRs in AC3-C (P = 0.001) and WT mice (P = 0.02), but not in AC3-I mice (Fig. 1C, P = 0.1). Langendorff-perfused hearts from WT, AC3-I, and AC3-C mice showed equivalent HRs in the absence of ISO (Fig. 1D). SAN cells generated spontaneous APs at similar rates (beats/min) regardless of genotype: WT (315 ± 12, n = 18), AC3-I (336 ± 9, n = 24), and AC3-C (325 ± 12, n = 19) (P = 0.4), similar to ex vivo and resting in vivo hearts. The difference in the effects of CaMKII inhibition on in vivo HRs during stress or ISO injection compared with HRs from resting mice, ex vivo HRs, and spontaneous AP rates in isolated SAN cells suggested that CaMKII is important for regulating sinus rhythm during fight or flight activation of the βAR signaling pathway.
Fig. 1.
HRs from AC3-I mice are slower than controls during stress in vivo, but not during rest or ex vivo. (A) HRs recorded in unanesthetized, physically restrained mice during echocardiography. In vivo HRs were significantly (P <0.001) slower in AC3-I mice than in controls (n = 9–12/group). (B and C) HRs recorded from ECG telemetered mice at rest (B) and after ISO injection (C) (0.4 mg/kg i.p.). In vivo HRs were significantly (P <0.05) slower in AC3-I mice compared with controls (n = 4–7/group) after ISO, but not at rest (P = 0.1). (D) Langendorff-perfused hearts from AC3-I and control mice (n = 5–6/group) beat at equivalent rates in the absence of ISO (P = 0.318). (E) ECGs recorded from Langendorff-perfused hearts at baseline and after 1 μM ISO. (F) ISO-HR response relationship in Langendorff-perfused hearts (n = 5–6/group). *, P <0.05 for AC3-I versus control hearts.
CaMKII Inhibition Reduces Maximal SAN Responses to ISO.
To quantify the contribution of CaMKII to HR increases by ISO, we measured HR responses over a range of ISO concentrations in Langendorff-perfused hearts from AC3-I and control mice (Fig. 1 E and F). AC3-I hearts had significantly reduced rate responses to ISO concentrations ≥ 10 nM compared with the control hearts. Protein kinase A (PKA) is activated by ISO, and CaMKII may be activated by PKA-dependent and -independent mechanisms (7). We treated Langendorff-perfused hearts with H-89, a PKA inhibitor, to test if the apparent contribution of CaMKII to increasing HR during ISO infusion required PKA. H-89 reduced ISO responses in all genotypes to a similar level (Fig. S1B), suggesting that CaMKII is activated “downstream” to PKA in this model.
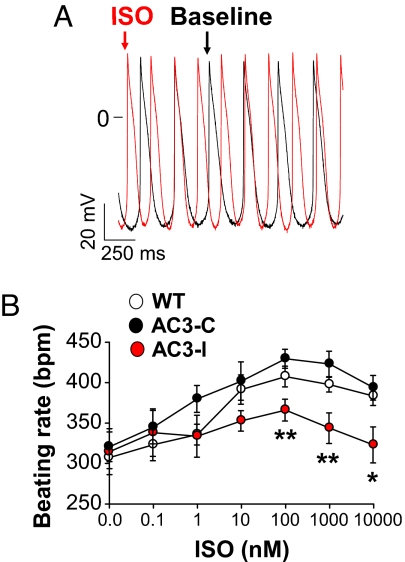
We next measured APs in SAN cells (Fig. 2A) exposed to the same ISO concentrations used in the excised heart studies to determine if SAN cells retain the CaMKII-mediated effects on HR seen in response to βAR stimulation. SAN cells from AC3-I mice showed significantly reduced AP frequencies at ISO concentrations >10 nM compared with SAN cells isolated from AC3-C and WT mice (Fig. 2B). Although the absolute AP frequencies in SAN cells (Fig. 2B) were significantly slower (P <0.01 for all of the groups) than in Langendorff-perfused hearts for each of the genotypes (Fig. 1D), the dynamic range of ISO stimulated rate increases (at ISO ≥10 nM) is similar in ex vivo hearts and in SAN cells (i.e., ≈40%). These findings showed that ISO responses in SAN cells and in ex vivo hearts were significantly reduced by AC3-I expression, consistent with the slower in vivo HRs during stress or ISO injection in AC3-I mice compared with controls.
Fig. 2.
SAN cell CaMKII inhibition reduces ISO rate responses. (A) Example recordings of APs in a WT SAN at baseline (black) and after 100 nM ISO (red). (B) SAN cell AP frequencies in response to a range of ISO concentrations. AC3-I SAN AP frequencies were significantly (*, P <0.05, **, P <0.01, ANOVA) slower than controls at each ISO concentration (n = 6–10 per data point).
AC3-I mice have myocardial CaMKII inhibition for their entire postnatal life, suggesting the possibility that the heart and SAN cell rate slowing effects in AC3-I hearts during ISO stimulation could have derived from unforeseen adaptations to chronic SAN CaMKII inhibition. To test if short-term (24 hr) CaMKII inhibition would result in a similar reduction in SAN rates in response to ISO, we infected WT SAN cells with adenovirus encoding a CaMKII inhibitory peptide, CaMKIIN (8). SAN cells infected by CaMKIIN had a significantly (P = 0.01) impaired response to ISO (1 μM) compared with WT SAN cells infected with enhanced green fluorescent protein (eGFP) encoding adenovirus alone (Fig. S2). We used a viral infection approach rather than a small molecule CaMKII inhibitor (e.g., KN-93) because the off-target effects of these drugs on ion channels potentially involved in SAN function occur at concentrations necessary to achieve CaMKII inhibition. Furthermore, these ion channel antagonist actions of KN-93 are not shared by the congener control agents (e.g., KN-92) (9, 10). We tested a cell membrane permeant, myristoylated CaMKII inhibitory peptide (M-AIP), and compared it with a myristoylated control peptide (M-AC3-C) developed for these studies. The M-AIP and M-AC3-C peptides caused similar depression of SAN cell automaticity (Fig. S3 A and B), but AIP inhibited CaMKII Thr-287 autophosphorylation in response to ISO whereas AC3-C did not (Fig. S3C), suggesting that rate slowing actions of M-AIP were not due to CaMKII inhibition. Our findings to this point showed that short-term and chronic SAN CaMKII inhibition by 2 different peptide inhibitors (AC3-I and CaMKIIN) significantly reduced ISO responses.
CaMKII Is Activated by ISO in SAN Cells.
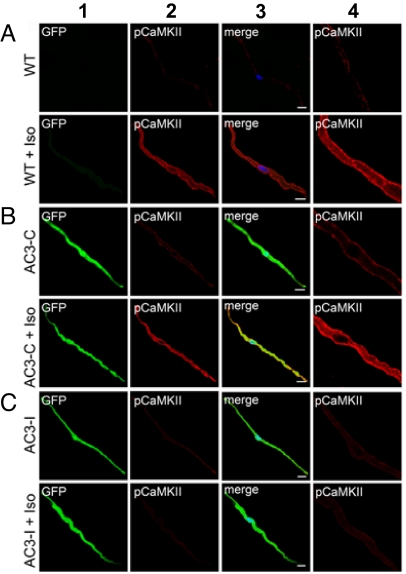
AC3-I and AC3-C peptides are expressed as a fusion product with eGFP in AC3-I and AC3-C transgenic mice. SAN cells from AC3-I and AC3-C mice showed easily detectable eGFP (Fig. 3 B and C, column 1), indicating that the cardiomyocyte-defining αMHC promoter was active in SAN. To determine if CaMKII was activated in SAN during βAR stimulation, we measured Thr-287 autophosphorylated CaMKII (pCaMKII), a marker of CaMKII activation (11), in SAN cells from WT, AC3-I, and AC3-C mice. pCaMKII was readily detected after ISO in WT and AC3-C (Fig. 3 A and B), but not in AC3-I SAN cells (Fig. 3C, column 2). pCaMKII was enriched in a subsarcolemmal distribution after ISO in control SAN cells, consistent with an earlier report (Fig. 3 A and B, column 4) (12). In contrast, pCaMKII was not evident in SAN cells from any of the mice in the absence of ISO stimulation. These data show CaMKII is a downstream signal in the βAR pathway in SAN cells and that AC3-I expression effectively prevents activation of CaMKII during βAR stimulation. These results support our physiological findings that CaMKII inhibition selectively reduces HRs only during βAR stimulation.
Fig. 3.
Representative immunofluorescence micrographs show ISO activates CaMKII in SAN cells isolated from WT (A) and AC3-C (B), but not from AC3-I mice (C) with SAN CaMKII inhibition. Columns are as follows: 1, eGFP (expressed in AC3-C and AC3-I SAN cells); 2, Thr 287 autophosphorylated, activated CaMKII (pCaMKII, red); 3, merge; 4, magnified images from column 2. (Scale bar, 10 μm.)
We measured PKA and CaMKII activity from SAN explants at baseline and in response to ISO at phospholamban (PLN) (13) using site and phosphor-specific antibodies against the PLN PKA site (Ser-16) and the PLN CaMKII site (Thr-17) (14). Basal PLN phosphorylation at Ser-16 was similar in all genotypes and ISO increased PLN Ser-16 phosphorylation to a similar extent in all genotypes (Fig. S4A), suggesting that PKA activity responses to ISO were similar in all genotypes. To test if PKA activity was also preserved in vivo, we injected mice with ISO. PKA activity was similar in SAN tissue excised from ISO injected mice from all genotypes (Fig. S1C), indicating that PKA activity responses to ISO were similar in vivo and in SAN tissue isolated from AC3-I, AC3-C, and WT mice. In contrast, PLN Thr-17 phosphorylation was significantly reduced in SAN explants from AC3-I compared with WT or AC3-C mice in response to ISO. The level of PLN phosphorylation at Thr-17 was increased by ISO in WT and AC3-C SAN, but not in AC3-I mice (Fig. S4B). Taken together, these data indicate that AC3-I expression selectively reduces CaMKII activity without disrupting PKA activity responses to ISO.
Increased Phase 4 Depolarization by ISO Requires CaMKII.
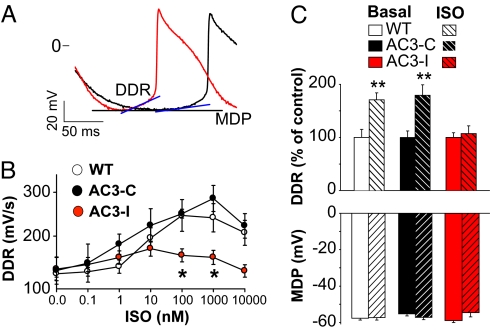
An increase in DDR, or so called phase 4 depolarization, is the membrane potential mechanism for SAN cells to increase HR (15). To test for a cellular mechanism to explain the HR slowing effects of CaMKII inhibition in SAN cells, we first measured DDR before and after ISO exposure. βAR stimulation increases DDR, leading to shortened diastolic intervals and faster SAN AP triggering (Fig. 4A). The DDR increased in step with increasing ISO, but DDR increases were significantly less in AC3-I compared with control SAN cells at ISO >10 nM (Fig. 4B), similar to the reduced rate responsiveness of AC3-I SAN cells to ISO (Fig. 2B). The maximum diastolic cell membrane potential (MDP) together with DDR determine the time necessary to reach the membrane potential threshold for AP triggering in SAN. The MDP was not different between AC3-I and control SAN (Fig. 4C), indicating that the dramatic loss of DDR responsiveness to ISO in AC3-I SAN was the cell membrane potential mechanism for slower HRs seen in AC3-I mice (Fig. 1) and in SAN cells isolated from AC3-I mice during ISO stimulation (Fig. 2).
Fig. 4.
Loss of DDR response to ISO in AC3-I SAN cells. (A) Expanded AP tracings from Fig. 2A show DDR increase after ISO (red) compared with baseline (black). (B) The relationship between DDR and ISO concentration. Control SAN cells have significant DDR increases with ISO (n = 5–7/group), but AC3-I SAN cells do not increase DDR with ISO. *, P <0.05 comparing all genotypes at each ISO concentration by ANOVA. (C) Summary data showing the maximum diastolic membrane potential (MDP) does not change during ISO (1 μM) increases in DDR and is not different between genotypes. Data for MDP and DDR in C are from 8–24 cells per group and include the cells in (B). **, P <0.01 for ISO versus baseline.
CaMKII Inhibition Reduces Diastolic Ca2+ Sparks During ISO.
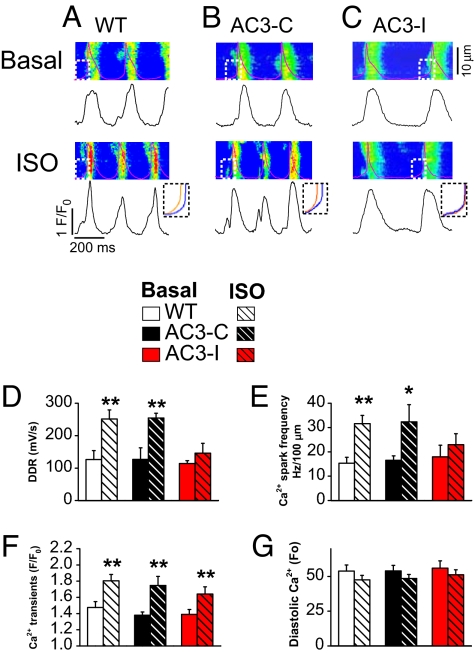
Diastolic SR Ca2+ release contributes to ISO-mediated increases in DDR by augmenting inward Na+/Ca2+ exchanger current (INCX) (16, 17) as part of a “Ca2+ clock” fight or flight cellular mechanism for increasing HR (18). We simultaneously measured cell membrane potential and Cai2+ in spontaneously beating SAN cells loaded with the Ca2+ indicator Rhod-2 acetoxymethyl ester using confocal line scan imaging at baseline and with ISO (Fig. 5A–C). The DDR (Fig. 5D) and diastolic Ca2+ spark frequency (Fig. 5E) both increased significantly with ISO in WT and AC3-C, but ISO failed to significantly increase either of these parameters in AC3-I SAN cells. The failure of AC3-I SAN cells to increase Ca2+ spark frequency with ISO contrasts with our earlier findings in ventricular myocytes (4), suggesting that CaMKII inhibition affects SR Ca2+ release differently in SAN cells and ventricular myocytes. The Ca2+ spark duration and spatial extent (Fig. S5 A and B) as well as the maximum rate of Cai2+ transient increase (Fig. S5C) were similar at baseline and after ISO in SAN cells from all genotypes, suggesting that CaMKII inhibition primarily reduced the frequency of spontaneous diastolic SR Ca2+ release without affecting maximum SR Ca2+ flux during systole. SAN cells from all genotypes had similar systolic Cai2+ amplitudes (Fig. 5F) and diastolic Cai2+, at baseline and after ISO (Fig. 5G). We measured the time to recovery of the Cai2+ transient, which partially reflects the rate of SR Ca2+ uptake. The Cai2+ transient recovered more slowly in AC3-I compared with control SAN cells at baseline and after ISO (Fig. S6). Taken together, these findings show that CaMKII inhibition reduces DDR diastolic SR Ca2+ release and slows SR Ca2+ uptake during ISO stimulation in SAN cells.
Fig. 5.
AC3-I SAN cells fail to increase diastolic Ca2+ spark frequency or DDR with ISO. (A–C) Representative line scan confocal images of Rhod-2 fluorescence and simultaneously recorded spontaneous APs (Upper) and spatially averaged Ca2+ transients (Lower) at baseline and after ISO (1 μM). (Insets) Superimposed DDR for each genotype, before and after ISO, corresponding to the dashed boxes marking the confocal images. (D–G) Summary data for DDR (D), Ca2+ spark frequency (E), Ca2+ transient (F), and diastolic Ca2+ (G) before and after ISO (1 μM) (n = 13–21 per group). *, P <0.05; **, P <0.01 compared with baseline.
SR Ca2+ Content Is Negatively Regulated by CaMKII Inhibition.
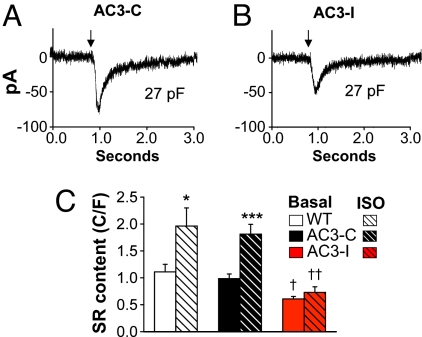
Increases in SR Ca2+ content enhance the probability of SR Ca2+ sparks in ventricular myocytes (19, 20), suggesting the hypothesis that reduced SR Ca2+ in AC3-I SAN cells could lead to the reduced diastolic SR Ca2+ release we observed during ISO stimulation (Fig. 5). We measured SR Ca2+ content in SAN cells by integrating INCX in response to a “spritz” of caffeine. INCX density was equivalent in SAN cells from AC3-I and control mice between −80 to +40 mV (Fig. S7A), suggesting that reduced HRs in AC3-I mice were not due to differences in INCX during phase 4 depolarization. SR Ca2+ content was significantly less in AC3-I SAN, compared with controls, at baseline and after ISO (Fig. 6). These findings show that CaMKII inhibition reduces or prevents SAN SR Ca2+ content increases during high βAR stimulation, a response that paralleled the actions of CaMKII inhibition to reduce ISO effects on the frequency of Ca2+ sparks, DDR, SAN cell AP frequencies and in vivo HRs. The finding that SR Ca2+ content was reduced in AC3-I SAN cells at baseline, in the absence of ISO—whereas DDR was similar at baseline in all genotypes—suggests that the differences in basal SR Ca2+ content between AC3-I and control SAN cells is insufficient to cause detectable differences in DDR. In contrast, the greater difference in SR Ca2+ content between AC3-I and control SAN cells after ISO was enough to cause measurable differences in DDR. Reduced SAN SR Ca2+ content in AC3-I mice is consistent with the concept that CaMKII inhibition slows SAN rates by affecting intracellular Ca2+ homeostasis.
Fig. 6.
SR Ca2+ content is reduced in AC3-I SAN cells. (A and B) Representative INCX recordings from an AC3-C (A) and AC3-I (B) SAN cells in response to a caffeine spritz (at arrow). SR Ca2+ content is calculated from the integral of INCX (see Materials and Methods for details). (C) Summary data for SAN SR Ca2+ content at baseline and after 1 μM ISO (n = 6–9/group). †, P <0.05 and ††, P <0.01 compared with WT and AC3-C; *, P <0.05 and ***, P <0.001 compared with basal.
Ryanodine Eliminates the Effect of CaMKII Inhibition on SAN Cell ISO Response.
We reasoned that if CaMKII inhibition limited SAN cell fight or flight physiology by slowing diastolic SR Ca2+ release, then severely reducing or eliminating SR Ca2+ release by an alternative means should significantly reduce or eliminate the differences in ISO responses between AC3-I and control SAN cells. Ryanodine is a selective ligand for ryanodine receptors and ryanodine causes a time and concentration-dependent slowing of SAN cell AP frequency (Fig. S8D). Ryanodine significantly and equivalently reduced the frequency of impulse formation in AC3-I and control SAN cells (Fig. S8 A and B). Interestingly, ryanodine treated SAN cells of all genotypes showed a reduction rather than an increase in AP frequencies after ISO, suggesting that in the absence of SR Ca2+ release ISO causes a CaMKII-independent reduction in net inward current that inhibits DDR. There were no differences in the ISO rates between ryanodine treated AC3-I and control SAN cells (Fig. S8C). These data show that SR Ca2+ release is critical to fight or flight responses in SAN and that SAN slowing effects of CaMKII inhibition are eliminated by ryanodine treatment.
CaMKII Inhibition Does Not Affect If During Phase 4 Depolarization Potentials.
Phase 4 depolarization is due to net inward current during SAN cell diastole (15). The funny current (If) is an important and well characterized inward current for increasing DDR during βAR stimulation (21). If was equivalent in SAN cells isolated from AC3-I and control mice at baseline and after ISO (Fig. S7 B and C), suggesting that HR slowing actions of CaMKII inhibition were independent of changes in If. Because If is activated by cAMP binding (22), the equivalent ISO responses in AC3-I and control SAN cells suggests that cAMP activity during βAR pathway stimulation is not affected by CaMKII inhibition, consistent with our findings that ISO-stimulated PKA activity was not reduced in SAN tissue from AC3-I mice compared with controls (Fig. S1C). These findings show that CaMKII inhibition slows fight or flight SAN responses to ISO by preventing increases in the DDR independently of If.
HR Slowing in AC3-I Mice Is Independent of ICa.
L-type Ca2+ current (ICa) in SAN cells is carried by pore-forming proteins that activate in a voltage dependent manner over phase 4 cell membrane potentials. Thus, ICa is the immediate source of Ca2+ necessary for filling the SR. ICa is increased by ISO, cAMP, and CaMKII (23), so these signals all have the potential to affect DDR and HR by augmenting SAN cell ICa. We measured ICa in SAN to determine if ICa was a downstream target for HR slowing in AC3-I mice. ICa was not significantly different in AC3-I and control SAN cells at baseline or after ISO (Fig. S9). These data indicate that the proximate mechanism for reduced DDR responses to ISO in AC3-I SAN cells is independent of ICa.
Discussion
Cardiac Pacemaker Cells in Health and Disease.
SAN failure is a significant cause of morbidity and mortality worldwide. More than $2 billion are spent annually on permanent, surgically implanted pacemakers in the United States alone (24). Furthermore, SAN failure is a frequent finding in patients with atrial fibrillation (25) and is associated with increased mortality in heart failure (26). Despite the clear importance of cardiac pacemaker cells for physiology and disease, critical knowledge gaps remain for understanding SAN cell signaling. Our study illustrates an unexpected role of CaMKII in regulating SAN responses to βAR stimulation and highlights the importance of (Cai2+) homeostasis for the physiology of contracting and pacing cardiomyocytes.
CaMKII Regulates SR Ca2+ Content in SAN Cells and Ventricular Myocytes.
We found SR Ca2+ content in SAN cells is comparable to SR Ca2+ content in ventricular myocytes when indexed to the sarcolemmal membrane area, supporting earlier work showing that ISO significantly increases SR Ca2+ content in both SAN cells (27) and in ventricular myocytes (4). CaMKII is activated during catecholamine stimulation in SAN cells (Fig. 3) and in ventricular myocytes, so CaMKII has the potential to contribute to fight or flight responses in SAN and contracting myocardium. However, it seems that ventricular myocytes and SAN myocytes use CaMKII signaling differently. CaMKII does not contribute to acute mechanical responses to ISO in ventricular myocytes, but CaMKII is essential for maintaining mechanical performance in ventricular myocytes during sustained (24 hr) catecholamine stimulation (28). Our new findings show that CaMKII is necessary for maximal βAR-mediated HR increases, and suggest a threshold of βAR activity is required to engage CaMKII for increasing HR. Excessive CaMKII activity induces pathological phenotypes in ventricular myocytes, including hypertrophy, loss of normal (Cai2+) homeostasis, arrhythmias, and death (29–31). It will be important to learn if excessive CaMKII activity contributes to similar maladaptive responses in SAN.
Are Antiarrhythmic and Bradycardic Actions of CaMKII Inhibition Similar?
CaMKII inhibition is antiarrhythmic in heart failure (29) and under conditions favoring (Cai2+) overload (32). The antiarrhythmic actions of CaMKII inhibition are due, at least in part, to protection against SR Ca2+ overload and suppression of SR Ca2+ release (33) that triggers arrhythmia-initiating afterdepolarizations due to inward INCX (32). CaMKII control over SR Ca2+ content and release thus appear to be central to the antiarrhythmic mechanism of CaMKII inhibition in ventricular myocytes and for the physiologic role of CaMKII to increase SAN cell excitability. Afterdepolarizations in ventricular myocytes and the phase 4 DDR of the SAN are both triggered by SR Ca2+ release and favored by catecholamine stimulation of CaMKII activity. CaMKII inhibition suppresses afterdepolarizations in ventricular myocytes (34) and slows the phase 4 DDR in SAN cells. Our findings indicate CaMKII plays a parallel role in coupling SR Ca2+ release to membrane excitability for proarrhythmic afterdepolarizations in contracting ventricular myocytes and for physiological fight or flight automaticity in SAN cells.
Physiological ISO-Mediated Chronotropy Relies on CaMKII.
Our findings in SAN cells and ex vivo and in vivo hearts show that a complete chronotropic response to ISO requires SAN CaMKII activity. In contrast to the role of CaMKII in determining HRs in vivo during stress (induced by physical restraint or ISO injection) and during ISO stimulation in ex vivo hearts or isolated SAN cells, CaMKII inhibition did not result in HR slowing at baseline. One study did show CaMKII inhibitors prevented spontaneous impulse formation in SAN cells in the absence of catecholamine stimulation (12). Based on our new findings with M-AC3-C, we presume that nonspecific actions of the CaMKII inhibitory agents available at the time of their study contributed to the discrepancy between our new findings and this earlier work.
Our ex vivo studies show that ≈50% of βAR stimulation induced increases in HR are CaMKII independent (Fig. 1F). In contrast, all chronotropic responses to ISO appeared to depend on normal SR Ca2+ filling and release because basal SAN automaticity was reduced by ryanodine, and ryanodine treatment caused HR slowing by ISO (Fig. S8). These findings complement other work emphasizing the critical importance of SR Ca2+ release for HR responses to ISO (3, 34). On the other hand, some investigators show a more modest effect of ryanodine treatment (35). The reasons for these differences are uncertain, but our findings that SAN slowing by ryanodine was both time and concentration dependent (Fig. S8D) suggest that differences in the experimental conditions may contribute to the discrepant experimental findings.
Our study does not preclude an important role for If in determining HR, but our findings do suggest that HR is regulated by multiple, potentially redundant mechanisms. We believe that reduction in HR by ISO in ryanodine-treated SAN cells indicates that diverse mechanisms of SAN automaticity are disrupted by loss of SR Ca2+ release and that If alone is insufficient to sustain physiological chronotropic responses to βAR stimulation in the absence of other depolarizing currents that require normal SR Ca2+ release.
Materials and Methods
Additional procedures can be found in SI Materials and Methods.
SAN Cell Isolation, Langendorff-Perfusion, and Electrophysiology.
See SI Materials and Methods for details.
Immunofluorescence and PLN Immunodetection Studies, Plasma Catecholamine, and PKA Activity Measurements.
See SI Materials and Methods for details.
SR Ca2+ Content Measurements.
SR Ca2+ content of SAN cells was measured by integrating the INCX (4) in response to a “spritz” of caffeine. See SI Materials and Methods for details.
Statistical Methods.
ANOVA was used for comparisons between 3 genotypes or for repeated measures, such as multiple ISO concentrations, in a single genotype. The Holm–Sidak test was used for post hoc comparison in cases where the P value of the ANOVA was <0.05. Paired or unpaired Student's t test was used to test the effects of interventions on a single genotype, as appropriate. Statistical significance was defined as P <0.05.
Supplementary Material
Acknowledgments.
We thank Shawn Roach at the University of Iowa for graphic design contributions. This work was funded by National Institutes of Health (NIH) Grants R01 HL 079031, R01 HL 62494, and R01 HL 70250 (to M.E.A.); NIH Grants R01 HL084583 and R01 HL083422, and the Pew Scholars Trust (to P.J.M.); NIH Grants R01 HL 090905 and AHA 0635056N (to L.-S.S.); NIH/NHLBI Grant R01 HL 089598 (to X.H.T.W.), and the University of Iowa Research Foundation. This work was also supported in part by the Fondation Leducq Alliance for CaMKII Signaling in Heart Disease.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.J.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806422106/DCSupplemental.
References
- 1.Herrmann S, Stieber J, Stockl G, Hofmann F, Ludwig A. HCN4 provides a “depolarization reserve” and is not required for heart rate acceleration in mice. EMBO J. 2007;26:4423–4432. doi: 10.1038/sj.emboj.7601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyashkov AE, et al. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ Res. 2007;100:1723–1731. doi: 10.1161/CIRCRESAHA.107.153676. [DOI] [PubMed] [Google Scholar]
- 3.Rigg L, Heath BM, Cui Y, Terrar DA. Localisation and functional significance of ryanodine receptors during beta-adrenoceptor stimulation in the guinea-pig sino-atrial node. Cardiovasc Res. 2000;48:254–264. doi: 10.1016/s0008-6363(00)00153-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 5.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. {beta}-Adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 6.Braun AP, Schulman H. A non-selective cation current activated via the multifunctional Ca2+-calmodulin-dependent protein kinase in human epithelial cells. J Physiol. 1995;488:37–55. doi: 10.1113/jphysiol.1995.sp020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bers DM. Going to cAMP just got more complicated. J Physio. 2007;583:415–416. doi: 10.1113/jphysiol.2007.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci USA. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson ME, et al. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharm Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- 10.Ledoux J, Chartier D, Leblanc N. Inhibitors of calmodulin-dependent protein kinase are nonspecific blockers of voltage-dependent K+ channels in vascular myocytes. J Pharmacol Exp Ther. 1999;290:1165–1174. [PubMed] [Google Scholar]
- 11.Kuret J, Schulman H. Mechanism of autophosphorylation of the multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1985;260:6427–6433. [PubMed] [Google Scholar]
- 12.Vinogradova TM, et al. Sinoatrial node pacemaker activity requires Ca2+/calmodulin- dependent protein kinase II activation. Circ Res. 2000;87:760–767. doi: 10.1161/01.res.87.9.760. [DOI] [PubMed] [Google Scholar]
- 13.Kranias EG, et al. The role of protein kinases and protein phosphatases in the regulation of cardiac sarcoplasmic reticulum function. Mol Cell Biochem. 1988;82:37–44. doi: 10.1007/BF00242513. [DOI] [PubMed] [Google Scholar]
- 14.Drago GA, Colyer J. Discrimination between two sites of phosphorylation on adjacent amino acids by phosphorylation site-specific antibodies to phospholamban. J Biol Chem. 1994;269:25073–25077. [PubMed] [Google Scholar]
- 15.Noble D. The initiation of the heart beat. Adv Sci. 1966;23:412–418. [PubMed] [Google Scholar]
- 16.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: Molecular partners in pacemaker regulation. Circ Res. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 17.Lancaster MK, Jones SA, Harrison SM, Boyett MR. Intracellular Ca2+ and pacemaking within the rabbit sinoatrial node: Heterogeneity of role and control. J Physiol. 2004;556:481–494. doi: 10.1113/jphysiol.2003.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maltsev VA, Lakatta EG. Normal heart rhythm is initiated and regulated by an intracellular calcium clock within pacemaker cells. Heart Lung Circ. 2007;16(5):335–348. doi: 10.1016/j.hlc.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisner DA, Choi HS, Diaz ME, O'Neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circ Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- 20.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- 21.DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 23.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 24.Lamas GA, et al. The mode selection trial (MOST) in sinus node dysfunction: Design, rationale, and baseline characteristics of the first 1000 patients. Am Heart J. 2000;140:541–551. doi: 10.1067/mhj.2000.109652. [DOI] [PubMed] [Google Scholar]
- 25.Hocini M, et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circ. 2003;108:1172–1175. doi: 10.1161/01.CIR.0000090685.13169.07. [DOI] [PubMed] [Google Scholar]
- 26.Saxon LA, Stevenson WG, Middlekauff HR, Stevenson LW. Increased risk of progressive hemodynamic deterioration in advanced heart failure patients requiring permanent pacemakers. Am Heart J. 1993;125:1306–1310. doi: 10.1016/0002-8703(93)90999-p. [DOI] [PubMed] [Google Scholar]
- 27.Vinogradova TM, Bogdanov KY, Lakatta EG. beta-Adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, et al. Sustained beta1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res. 2004;95:798–806. doi: 10.1161/01.RES.0000145361.50017.aa. [DOI] [PubMed] [Google Scholar]
- 29.Khoo MS, et al. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation. 2006;114:1352–1359. doi: 10.1161/CIRCULATIONAHA.106.644583. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 31.Erickson JR, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Roden DM, Anderson ME. Calmodulin kinase inhibition prevents development of the arrhythmogenic transient inward current. Circ Res. 1999;84:906–912. doi: 10.1161/01.res.84.8.906. [DOI] [PubMed] [Google Scholar]
- 33.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+-calmodulin-dependent protein kinase modulates RyR2 phosphorylation and SR Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 35.Honjo H, et al. Sarcoplasmic reticulum Ca2+ release is not a dominating factor in sinoatrial node pacemaker activity. Circ Res. 2003;92:E41–E44. doi: 10.1161/01.res.0000055904.21974.be. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.