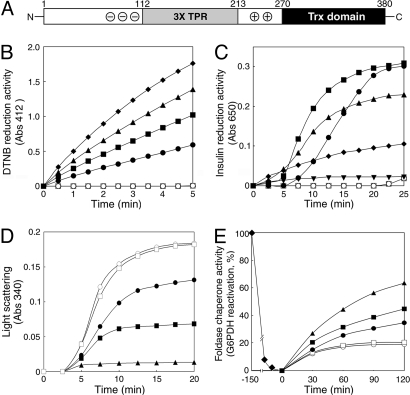
Fig. 1.
Multiple functions of AtTDX as a disulfide reductase, a foldase chaperone, and a holdase chaperone. (A) A scheme of the bipartite AtTDX protein structure. The N-terminal TPR domain is flanked by acidic (⊝) and basic (⊕) residues and the C terminus bears a Trx motif. (B and C) Disulfide reduction activity of AtTDX. Reduction of disulfide bonds using either 5 mM DTNB in the presence of NADPH and Trx reductase (B) or 30 μM insulin in the presence of 0.5 mM DTT (C) was measured by absorbance changes at A412 (B) and A650 (C), respectively. Concentrations of AtTDX used were 5 μM (●), 10 μM (■), 20 μM (▴), 30 μM (□), and 60 μM (▾). (D) Holdase chaperone activity of AtTDX. Thermal aggregation of 1.8 μM MDH was examined, with the molar ratios of AtTDX to MDH set at 1:1 (●), 3:1 (■), and 6:1 (▴) at 43 °C. (E) Foldase chaperone activity of AtTDX. The Cys-free form of G6PDH (10 μM) was denatured in 6 M urea for 2.5 h (□) and then refolded in a renaturation buffer in the presence of 5 μM (●), 10 μM (■), or 30 μM (▴) AtTDX. Activity of native G6PDH was set to 100%. Reactions using 30 μM ovalbumin (□) instead of AtTDX or lacking both AtTDX and ovalbumin (○) were used as controls.