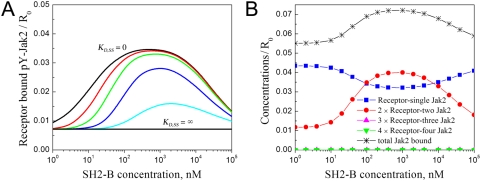
Figure 4. SH2-Bβ dimerization coordinates the formation of macro-complexes containing two Jak2 molecules bound to GH-dimerized receptors.
Steady-state calculations were performed using the Simplified Cellular Model and the same parameter values as in Figure 3, except with 10 nM GH stimulation and varying SH2-Bβ concentration. (A) Receptor-bound, phosphorylated Jak2 (Y2∼P), for various values of the SH2-Bβ dimerization affinity. The extreme cases of KD,SS equal to zero and infinity correspond to irreversible and no dimerization, respectively; intermediate KD,SS values are 10 nM, 100 nM, 1 µM, and 10 µM. (B) Analysis of receptor/Jak2 complexes, with KD,SS = 100 nM. SH2-Bβ dimerization coordinates the binding of two Jak2 molecules to dimerized receptors, while affecting overall receptor/Jak2 binding only modestly. Complexes containing more than two Jak2 molecules (e.g., J(RLR)JS 2 J) are rare.