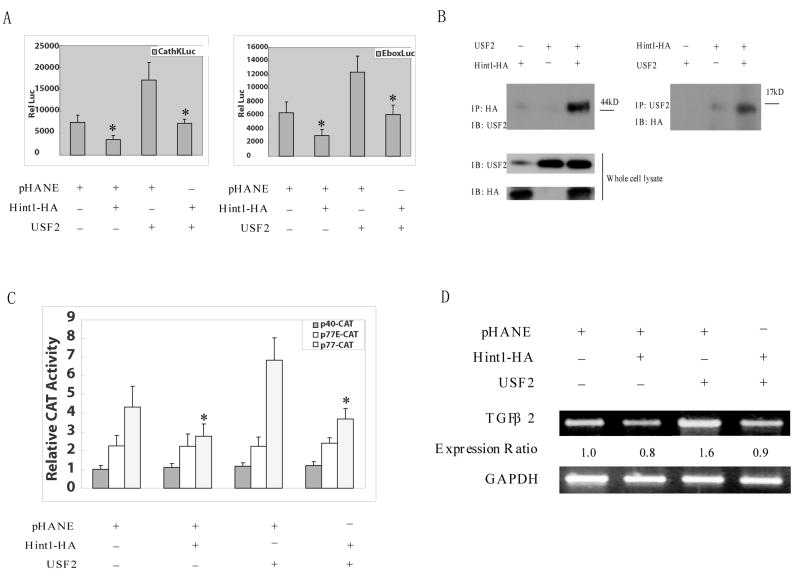
Figure 4. HINT1 inhibits USF2/E-box related transcription factor activity and TGFβ2 expression in HepG2 cells.
(A), The p-cathepsin K-LUC or pE-box-LUC, and CMV-β-gal reporter plasmid DNAs, were co-transfected into HepG2 cells. pHANE, pHA-Hint1 and pUSF2 plasmid DNAs were also co-transfected, as indicated. Luciferase activity was assayed 36 hours later and corrected for β-gal activity, as described in Fig. 3A. All assays were done in triplicate and a repeat study gave similar results. (B), HepG2 cells were transfected with pCMV-USF2 or pHA-Hint1 plasmid DNAs as indicated. After 48 hours, whole cell protein extracts were prepared and were immunoprecipitated with an anti-HA monoclonal affinity gel (left panel) or an anti-USF2 polyclonal antibody together with protein G plus-agarose (right panel), at 4°C for 18 hours. The immunoprecipitates were washed with cold lysis buffer five times, and then analyzed on Western blots with an anti-USF2 polyclonal antibody or an anti-HA monoclonal antibody to detect the USF2 or the HA tagged HINT1 proteins in the immunocomplexes and in the whole cell lysate (bottom panel), as indicated. (C), HepG2 cells were transfected with wild type (p77-CAT) or mutant (p40-CAT, p77E-CAT) TGF-β2 promoter/CAT reporter plasmid DNAs together with the CMV-β-gal reporter plasmids DNA. At 36 hour post transfection, whole cell extracts were prepared, and CAT activity was assayed and normalized for β-gal activity. The results are expressed as the CAT activities of the pβ2-77 or pβ2-77E relative to the activity of the pβ2-40 construct. All assays were done in triplicate and a repeat study gave similar results. (D), pHA-Hint1 or the pCMV-USF2 plasmid DNAs were transfected into HepG2 cells, as indicated. At 48 hours post transfection, whole cell RNA was extracted, and semi-quantitative RT-PCR was performed using specific TGF β2 and GAPDH primer sets, as described in Fig. 4D. Assays were repeated twice and gave similar results.