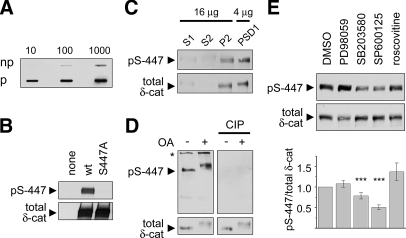
Fig. 6.
δ-Catenin serine 447 is phosphorylated by JNK in neurons. A, affinity-purified antibody raised against δ-catenin Ser(P)-447 (pS-447) was tested against the phosphopeptide used for immunization (p) and an identical non-phosphorylated peptide (np) in a slot blot (10, 100, or 1000 ng of peptide). B, GFP-tagged wt δ-catenin or a mutant with serine 447 substituted by alanine (S447A) was expressed in HEK293 cells and immunoblotted with pS-447 phosphoantibody or GFP antibody. C, immunoblot of rat brain fractions with pS-447 and total δ-catenin antibodies. Brain extracts were fractionated into postnuclear supernatant (S1), cytosol plus light membranes (S2), crude synaptosomal fraction (P2), and synaptosomes extracted with 0.5% Triton X-100 (PSD1). D, extracts from untreated or OA-treated (100 nm, 1 h) cortical cultures (DIV25) were probed with antibodies as indicated. CIP treatment removed pS-447 phosphoantibody signal but spared total δ-catenin (δ-cat). The asterisk denotes a cross-reactive band detected by the phosphoantibody. E, hippocampal cultures (DIV23–27) treated with DMSO or kinase inhibitors were immunoblotted with antibodies to Ser(P)-447 δ-catenin and total δ-catenin. Histogram shows immunoblot intensity of Ser(P)-447/total δ-catenin normalized to DMSO control (mean ± S.E. of seven independent experiments). Statistical analysis was performed by two-sided t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.