Abstract
Objective
We wanted to evaluate the therapeutic efficacy of the percutaneous balloon dilatation and large profile catheter maintenance method for the management of patients with anastomotic biliary strictures following liver transplant.
Materials and Methods
From May 1999 to June 2003, 12 patients with symptomatic benign biliary stricture complicated by liver transplantation were treated with the percutaneous balloon dilatation and large profile catheter maintenance method (1-6 months). The patients were eight males and four females, and their ages ranged from 20 to 62 years (mean age: 44 years). Ten patients underwent living donor liver transplantation and two underwent cadaveric liver transplantation. Postoperative biliary strictures occurred from two to 21 months (mean age: 18 months) after liver transplantation.
Results
The initial technical success rate was 92%. Patency of the bile duct was preserved for eight to 40 months (mean period: 19 months) in 10 of 12 (84%) patients. When reviewing two patients (17%), secondary balloon dilatations were needed for treating the delayed recurrence of biliary stricture. In one patient, no recurrent stenosis was seen during the further 10 months follow-up after secondary balloon dilatation. Another patient did not response to secondary balloon dilatation, and he was treated by surgery. Eleven of 12 patients (92%) showed good biliary patency for 8-40 months (mean period: 19 months) of follow-up.
Conclusion
The percutaneous balloon dilatation and large profile catheter maintenance method is an effective therapeutic alternative for the treatment of most biliary strictures that complicate liver transplantation. It has a high success rate and it should be considered before surgery.
Keywords: Liver, Bile ducts, Bile ducts abnormalities, Intervention
Liver transplantation is a widely accepted treatment for end-stage liver disease. Despite the improvements that have been made in organ preservation technology, the refinement of surgical technique and the advances in immunosuppressive strategies, postoperative biliary stricture is still a significant cause of morbidity and mortality after liver transplantation (1). According to the recently published literature, biliary complications occur in approximately 10% to 50% of all cases of liver transplantation (2, 3). In particular, bile leakage and anastomotic stricture are the most common complications. End-to-end choledochocholedochostomy is a usual pattern of anastomosis between the donor's and recipient's common bile ducts (CBD) during liver transplantation, and anastomotic site stricture occurs in about 10% of these patients (4). Patients with benign postoperative biliary strictures have a long average life expectancy. Therefore, the major goal of percutaneous treatment is to maintain the long-term patency of biliary strictures with balloon dilatation and/or stent placement. Recurrent stenosis after balloon dilatation for treating benign biliary stricture occurs in 29 58% of the cases (5, 6). During the past few years, several reports have advocated performing endoscopic or percutaneous stenting as an effective method with high success rate for treating anastomotic strictures (7, 8). This was not the case in our series despite that all procedures were performed by experienced interventional endoscopists and radiologists. The overall success rate for treating anastomotic stricture by performing dilatation or stent insertion was 27% and none of the patients who required more than one stent insertion displayed resolution of their problems. The 21% failure rate for endoscopic retrograde cholangiopancreatography (ERCP) was in keeping with the reported rates (9-60%) (9). Acute pancreatitis (21%) and stent blockage (30%) were the main complications. Accordingly, we suggest performing percutaneous balloon dilatation with temporary maintenance of a large profile catheter across the benign biliary stricture as an alternative method to treat resistant and refractory lesions. We report here on the short-term results of using this technique for the treatment of symptomatic benign postoperative biliary strictures in 12 liver transplantation patients.
MATERIALS AND METHODS
Patients
From May 1999 to June 2003, a total of 104 consecutive patients suffering with hepatic failure underwent liver transplantation at our institution. Of these, 12 patients who experienced symptomatic benign postoperative biliary stricture after liver transplantation were treated with the balloon dilatation and large profile catheter-maintenance method. The patients were eight males and four females, and their ages ranged from 20 to 62 years (mean age: 44 years). The underlying causes of the native liver failure that required liver transplantation included liver cirrhosis due to hepatitis B (n = 10), fulminant hepatitis (n = 1), and Wilson's disease (n = 1). Ten patients underwent living donor liver transplantation and two patients underwent cadaveric liver transplantation. The postoperative biliary strictures occurred from two to 21 months (mean period: 18 months) after liver transplantation.
The types of biliary anastomosis were end-to-end choledocho-choledochostomy in nine patients and choledocho-jejunostomy or hepaticojejunostomy with a Roux-en-Y in three patients. The sites of biliary strictures were all anastomotic and two patients had complex biliary strictures involving both the anterior and posterior segmental ducts.
All the patients initially presented with laboratory and clinical findings suggestive of biliary obstruction, and this was initially diagnosed from routine surveillance by the liver transplant service. The patients were given a broadspectrum antibiotic Cefotaxime (cefotaxime sodium; Chong Kun Dang Pharmaceutical Corp., Seoul, Korea), and then they were transferred to the interventional radiology department for percutaneous transhepatic biliary drainage (PTBD).
Balloon-Dilatation Procedure
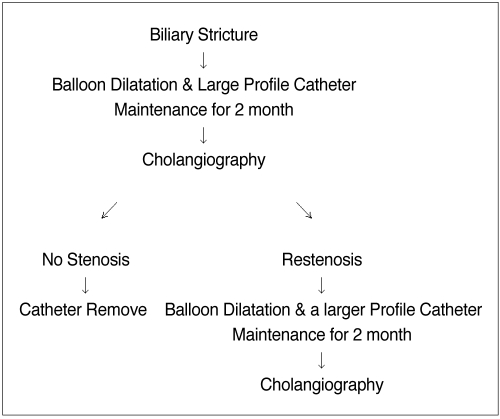
All the patients were given local anaesthesia before their procedures, i.e., 10 mL of 2% lidocaine (lidocain; Kwang Myung Pharmaceutical Corp., Seoul, Korea). Demerol (50 mg) (pethidine hydrochloride, Kuk Dong Pharmaceutical Corp., Soo Won, Korea) was given intravenously to relieve pain. A 8.5 Fr external or internal-external biliary drainage catheter (Cook, Bloomington, IN) was inserted into the biliary tree after cholangiography was done by percutaneous transhepatic access. If a missing duct was detected on the cholangiogram, then an additional puncture was done (Figs. 1A-D). Four to six weeks later, the existing biliary drainage catheter was exchanged for a vascular sheath; this was done over a standard 0.035-inch guidewire (Radifocus M, Terumo, Tokyo, Japan). The cholangiography was performed through the sheath and we evaluated the grade of biliary stricture. Different types of 5 Fr catheters (Angled Taper or Cobra or Shepherd Hook, Terumo, Tokyo, Japan) were advanced along the guidewire to cross the stricture site and the wire was exchanged for a stiff one (Amplatz Super Stiff, Boston Scientific Medi-Tech, Watertown, MA). The balloon dilatation catheter (Courier; Boston Scientific Medi-Tech, Watertown, MA) was then placed across the stricture and inflated during 10-30 second per session until the stenosis disappeared (Fig. 1B). A 6-8 mm × 40 mm balloon was used for dilatation of the strictures that extended into the second order branch intrahepatic ducts, and a 10-12 mm × 40 mm balloon was used for the dilatation of the extrahepatic stricture. The tracts were dilated in a stepwise fashion with dilators (Cook, Bloomington, IN) to accept a biliary drainage catheter; these dialators ranged in size from the 8 to 16 Fr. An internal-external biliary drainage catheter (14-18F) (Flexima, Cook, Bloomington, IN) or a percutaneous transhepatic cholangiographic catheter (Akita Sumitomo Bakelite Corp., Tokyo, Japan) was chosen to over-expand the normal duct diameter by 10-20% and it was inserted for internal-external biliary drainage for a period ranging from 30 to 90 days (average period: 60 days) after balloon dilatation (Figs. 2A-D). The dilation procedure was generally performed during a period of 1-2 week with one to three sessions (mean number of sessions: two sessions) for each treatment. The numbers of dilation sessions were determined by the subsequent cholangiographic findings that were obtained before a repeated dilatation procedure. If cholangiography showed no restenosis, the catheter was removed. If bile duct narrowing was still present, balloon dilatation was then repeated with a balloon sized up to 25-30% larger than the estimated diameter of the duct being dilated. The balloon was inflated to approximately 6-8 atmospheres (Encore 26 Inflation Device; Boston Scientific Tullamore Limited, Galway, Ireland). After percutaneous balloon dilatation, the catheter was maintained for over two month, and then cholangiography was performed. If the follow-up cholangiogram showed a residual stenosis less than 20% and drainage of the contrast material into the small bowel within 30 seconds, then we considered the procedure to be technically successful and we removed the large profile catheter. When the residual stenosis was more than 20% or drainage of the contrast material into small bowel took more than 30 seconds, a larger diameter internal-external biliary drainage catheter was then inserted for a period ranging from 30 to 90 days after balloon dilatation (Fig. 3).
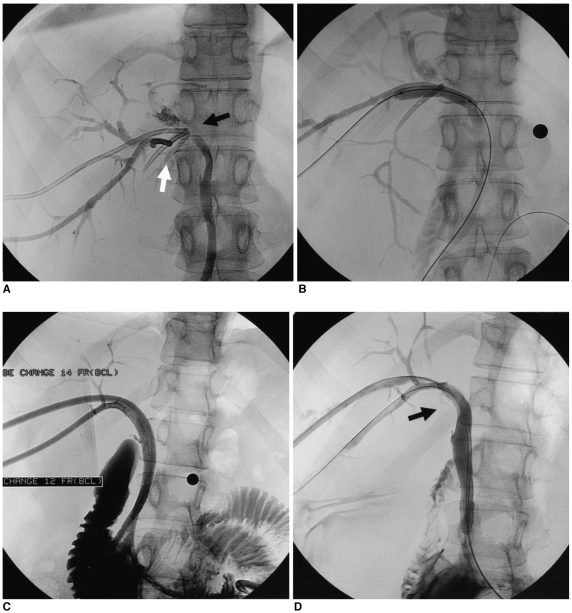
Fig. 1.
A 57-year-old male with choledocho-choledochostomy after living donor liver transplantation.
A. The cholangiography obtained after transhepatic insertion of a biliary drainage catheter shows a biliary anastomotic stricture, which divided the fifth and eighth segment ducts from the sixth and seventh segment ducts (white and black arrow).
B. An 8 mm diameter balloon catheter was positioned through the anastomotic stricture.
C. Two internal-external biliary drainage catheters (12 and 14 F) were inserted after biloplasty.
D. The cholangiogram after the large profile catheter maintenance method (3 months) shows patent bile ducts (arrow), with excellent flow of contrast medium into the duodenal loop.
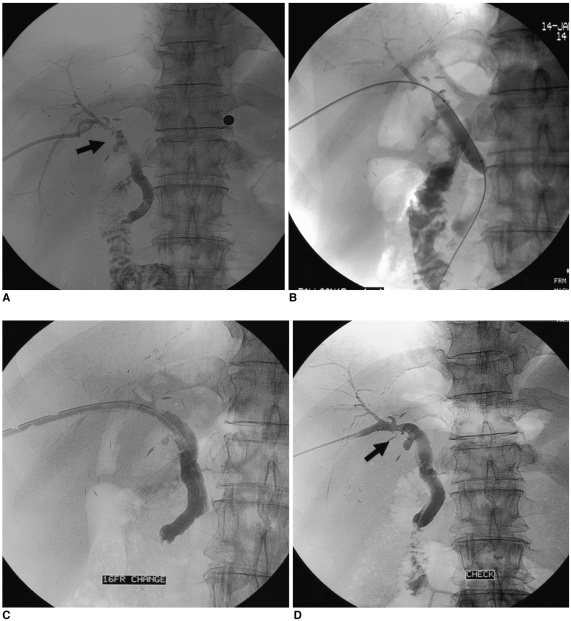
Fig. 2.
A 56-year-old man with choledocho-choledochostomy after living donor liver transplantation.
A. The cholangiography obtained during transhepatic insertion of a biliary drainage catheter shows a biliary anastomotic stricture (arrow).
B. An 8 mm diameter balloon catheters was positioned through the anastomotic stricture.
C. A percutaneous transhepatic cholangiographic catheter (16 F) was inserted after biloplasty.
D. The cholangiogram after the large profile catheter maintenance method (2 months) shows patent bile ducts (arrow), with excellent flow of contrast medium into the duodenal loop.
Fig. 3.
Flow diagram showing the balloon dilatation and large profile catheter maintenance method following liver transplantation.
Mechanical lithotripsy were performed by using a 10-mm diameter balloon catheter (Courier; Boston Scientific Medi-Tech, Watertown, MA) and/or saline flushing in two patients who displayed lithiasis and/or sludge in order to clear the biliary tree. Balloon sphincteroplasty was performed with a 10-mm balloon catheter prior to attempting the passage of sludge and stones into the duodenum. To avoid pancreatitis, pharmacologic protection was performed with 0.1 mg ocreotide, t.i.d (Sandostatin, Norvatis Korea, Seoul, Korea) three days before and after treatment for the patients with an intact sphincter of Oddi.
Follow-up
All of the patients' clinical and radiologic data were recorded on standard forms. The follow-up evaluation included assessment of serum bilirubin and the liver enzyme levels (alkaline phosphatase, aspartate aminotransferase and alanine aminotransferase); these assessments were performed before percutaneous drainage and before and after balloon dilatation. Clinical assessment and the serum bilirubin and liver enzyme assays were also performed at one, three, six and nine months after balloon dilatation. Between these assessments, monthly control examinations were also performed via telephone interviews with the patients or their family.
Complications such as cholangitis or pancreatitis were diagnosed by the clinical sign and symptoms and by laboratory test, and when indicated by the imaging studies. When the patients had signs of jaundice, then ultrasonography or computed tomography (CT) of the liver was performed to determine whether the jaundice was caused by dilated intrahepatic ducts and if repeated intervention was indicated.
This study was approved by our institutional review board. A written informed consent was obtained from all patients after the nature of the procedures was fully explained.
Study Endpoints and Definition
The major study endpoints were the assessment of technical success, the safety of balloon dilatation, the biliary patency and patient survival.
The intervention was considered technically successful when we observed complete or essentially complete (< 20% residual stenosis) restoration of the anastomosis and/or bile duct lumen diameter, and there was drainage of contrast material into the small bowel within 30 second after cholangiography. Safety was judged based on the incidence of complications. Events directly related to balloon dilatation that resulted in admission to the hospital for therapy (if the patient had been discharged), an unexpected increase in the level of care, prolonged hospitalization, permanent adverse sequalae or death were classified as major complications. Minor complications were events that required nominal therapy or observation and these events resulted in no sequalae.
The definition of a balloon dilatation with lasting patency and the clinical successful treatment during the follow-up period was the absence of recurrent biliary obstruction. Primary patency of the balloon dilatation was defined as the time interval between the initial placement and recurrence of obstruction. If there was no evidence of obstruction during the patient's life, the patency period was considered equal to the survival period, but this was censored. Survival of the patients was defined as the time interval between the initial placement and the patient's death.
Statistical Analysis
Statistical analysis for significance was performed using a paired t-test. Significance was accepted if the p values were < 0.05. All the patients' radiology reports and cholangiograms were reviewed. An electronic chart review of all the procedures was performed, and this included the operative notes, discharge reports, pathology reports, cultures and laboratory data. Additional follow-up was directly obtained from the clinical service in all cases.
RESULTS
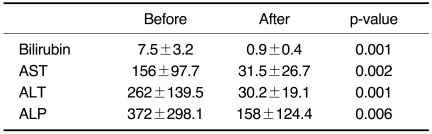
We obtained follow-up for all the cases, and technical success of balloon dilatation was achieved for 11 of 12 (92%) patients after percutaneous balloon dilatation with the large profile catheter maintenance method. All the patients showed clinical improvement and relief of jaundice (the average bilirubin level was 7.5 mg/dL ± 3.2 before procedure and 0.9 mg/dL ± 0.4 a month after the procedure). In the all patients at least one month after catheter removal, the serum bilirubin levels decreased to below 2.0 mg/dL in all cases (Table 1).
Table 1.
Laboratory Findings before and after Balloon Dilatation and the Large Profile Maintenance Method following Liver Transplantation
Note.-AST = aspartate aminotransferase, ALT = alanine aminotransferase, ALP = alkaline phosphatase.
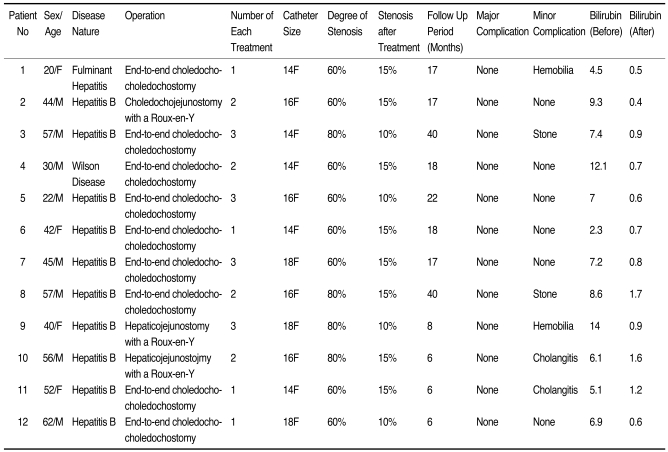
Clinical follow-up was completed in 12 patients from eight to 40 months (mean period: 19 months) after catheter removal (Table 2). Patency of the bile duct was preserved for 8-40 months (mean period: 19 months) in 10 of 12 (84%) patients. In two patients, recurrence of biliary strictures were seen at six and eight months, respectively, after the last percutaneous balloon dilatation. Among these two, one patient underwent additional balloon dilatation and he showed no recurrence of stenosis on the 10-month follow up. The other patient did not respond to balloon dilatation and he was immediately treated by surgery.
Table 2.
Clinical Data and Results of the Balloon Dilatation and Large Profile Catheter Maintenance Method following Liver Transplantation
Doppler ultrasound scanning was performed in all patients for evaluating the abnormalities in the hepatic arteries. Three patients showed hepatic artery stenosis on their Doppler study and two were treated with percutaneous angioplasty. The other patient did not have angioplasty performed due to patient's poor general condition.
The presence of biliary stones had no influence on the success of treatment. Two of 12 patients had biliary stones associated with bile duct stenosis. However, these two patients were disease-free after percutaneous balloon dilatation and clearing of the biliary tract.
There were no important complications; minor complications occurred in three patients and they presented with acute cholangitis. This was immediately treated by antibiotics and these patients showed immediate improvement. Two patients had hemobilia immediately after balloon dilatation, but this spontaneously resolved after 3-7 days.
DISCUSSION
Biliary tract complications are well-known causes of the substantial morbidity and mortality that occur after hepatic transplantation (10, 11). Biliary strictures in the liver transplant population can be classified as anastomotic and nonanastomotic (including hilar) (11). The anastomotic strictures observed after transplantation tend to be tough fibrotic strictures that are the result of scarring and retraction, and this may be related to peribiliary ischemia. Nonanastomotic, or hilar and intrahepatic biliary strictures can be multifactorial and they are usually related to hepatic artery thrombosis, a prolonged donor cold ischemic time, chronic rejection, ascending cholangitis and recurrence of pre-transplantation liver disease (12, 13).
Hepatic artery occlusion has been reported to be the most frequent cause of biliary strictures (13, 14). The bile duct is normally fed by the hepatic and gastroduodenal arteries and their collateral vessels. During liver transplantation, the collateral vessels are excluded from the hepatic artery anastomosis; this alone supplies the donor liver. In this condition, a minimal decrease of the hepatic artery flow may cause ischemic stricture of the bile duct that is anastomotic and/or nonanastomotic. Greif et al. (15) have reported that hepatic artery thrombosis was found in 83% of the patients with nonanastomotic bile duct strictures. In our study, the patients with intra-hepatic and nonanastomotic bile duct strictures showed decreased hepatic artery flow on their color Doppler studies. In our total cases, three patients had hepatic artery stenosis. Two patients were treated with percutaneous angioplasty and they showed a good response to subsequent biliary balloon dilatation due to a restored blood supply to the biliary tract. Another patient was not treated with percutaneous angioplasty, and the bile duct stenosis did not response to subsequent balloon dilatation.
Although no clear therapeutic algorithm has currently been reported, there have been many approaches to treat biliary stenosis, including surgical, endoscopic or percutaneous techniques. With the development of the interventional radiological techniques, percutaneous balloon dilatation and stent placement have become more accepted techniques for the initial management of the biliary strictures seen in liver transplant patients. Most series have reported disappointing results with less than a 50% patency rate at 1-2 years (11, 16). However, some series have reported better results with using percutaneous balloon dilatation for the nonanastomotic stricture than for the anastomotic strictures (17, 18). Zajko et al. (17) reported an 89% initial success rate and 70% 6-year success rate for percutaneous balloon dilatation, which was comparable to that for surgery. To obtain these results, Zajko et al. used multiple dilatations (up to six times per patient; mean number of dilations: three) during a 1-2 week period.
The clinical usefulness of performing metallic stenting for treating biliary strictures after liver transplantation is not well established. Culp et al. (19) and Diamond et al. (20) have used metallic stents for patients in whom the initial balloon dilatation failed. Culp et al. (19) have reported an 88% secondary patency rate for the metallic stents in liver transplant patients at five years. Unfortunately, the repetitive procedures required to maintain patency have lead to increased complications and even deaths (19). Several early reports found expandable metallic stents to be a useful adjunct for patients with refractory biliary strictures (21, 22). The initial technical success rate and short-term primary patency rate were nearly 85% at six months (22).
The major disadvantage of metallic stents compromises any subsequent surgical approach as they become embedded in the bile duct wall. However, if percutaneous balloon dilatation fails, there is no influence on the subsequent surgical revision. In some patients who suffered with exceptionally refractory strictures, an expandable metallic stent was found to be useful to alleviate their biliary obstruction until death from other causes as long as two years after stent placement (23). The primary patency of the stents may decrease to less than 50% at 12-18 months, and this may decrease to as low as 0% at five years (19). Therefore, metallic stents are not a long-term solution for the biliary strictures in most liver transplant patients.
Petersen et al. (21) have reported on using temporary covered stent placement for the treatment of benign biliary stricture in liver transplant patients. But the use of covered stents is limited to treating multiple strictures because it occludes the side branch ducts.
The principal advantage of our method is that repetitive stepwise larger size balloon dilations over a 1-2 weeks period can achieve adequate bile duct dilatation with a low risk of ductal rupture and bleeding. The dilated ductal and periductal tissue can be stabilized during the interval between each session and the mechanical stress per session can be divided up and diminished. The catheter placed across the stricture does not get incorporated into bile duct wall, and long-term maintenance (2-6 months) of the larger profile catheter (18 Fr) has a same effect as stenting as well as that the catheter can be easily removed when the biliary patency is stable.
Although some studies (10, 15, 17, 24) have reported that there were no complications after percutaneous procedures, the well-known complications of the percutaneous approach are hemobilia, bleeding, cholangitis, pancreatitis, biliary fistula and bile duct perforation. The overall complication rate was 42% in our series and such complications as acute cholangitis were treated with antibiotics; this resolved spontaneously in all of cases. In our study, the rate of cholangitis was high (25%), and this was possibly due to ascending infection, and this ascending infection was independent from the type of anastomosis.
In this series on patients treated with track dilation, there was an overall lower complication rate, which was comparable to the 14.5-22% reported in the other previous percutaneous transhepatic choledochoscopy (PTCS) series (25-29). As a result of progressive dilation of the biliary track, the complication and mortality rates were found to be significantly reduced (26, 29). We encountered no massive bleeding or bile duct perforation, the same as in the other series (27-29). This could be related to the practice of waiting 4-6 weeks for the track to fully mature before performing dilatation.
This study has some limitations. First, we report only the interim results of the balloon dilatation and large profile catheter maintenance method. Therefore, long-term follow-up after complete treatment is necessary. Second, because no clear therapeutic algorithm has been reported to date, we used our own criteria to assess the therapeutic effect. Third, our study group was small, and additional studies are needed to further assess the role of this method.
In conclusion, repetitive gradual balloon dilatations and the large profile catheter maintenance method were a highly effective and successful therapeutic alternative for the treatment of biliary complication after liver transplantation. We think this method should always be considered before surgical intervention. A larger series with a longer follow-up period is required to better determine the therapeutic efficacy of this new technique for the treatment of biliary strictures.
Acknowledgments
We are grateful for the editorial assistance provided by Yong-Whee Bahk, MD and Kyung Rae Kim, MD.
References
- 1.Stratta RJ, Wood RP, Langnas AN, Hollins RR, Bruder KJ, Donovan JP, et al. Diagnosis and treatment of biliary tract complications after orthotopic liver transplantation. Surgery. 1989;106:675–683. [PubMed] [Google Scholar]
- 2.Rabkin JM, Orloff SL, Reed MH, Wheeler LJ, Corless CL, Benner KG, et al. Biliary tract complications of side-to-side without T tube versus end-to-end with or without T tube choledochocholedochostomy in liver transplant recipients. Transplantation. 1998;65:193–199. doi: 10.1097/00007890-199801270-00008. [DOI] [PubMed] [Google Scholar]
- 3.Thethy S, Thomson B, Pleass H, Wigmore SJ, Madhavan K, Akyol M, et al. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647–653. doi: 10.1111/j.1399-0012.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 4.Gholson CF, Zibari G, McDonald JC. Endoscopic diagnosis and management of biliary complications following orthotopic liver transplantation. Dig Dis Sci. 1996;41:1045–1053. doi: 10.1007/BF02088217. [DOI] [PubMed] [Google Scholar]
- 5.Citron SJ, Martin LG. Benign biliary strictures: treatment with percutaneous cholangioplasty. Radiology. 1991;178:339–341. doi: 10.1148/radiology.178.2.1987589. [DOI] [PubMed] [Google Scholar]
- 6.Mueller PR, Ferrucci JT, Jr, Teplick SK, vanSonnenberg E, Haskin PH, Butch RJ, et al. Biliary stent endoprosthesis: analysis of complications in 113 patients. Radiology. 1985;156:637–639. doi: 10.1148/radiology.156.3.4023221. [DOI] [PubMed] [Google Scholar]
- 7.Klein AS, Savader S, Burdick JF, Fair J, Mitchell M, Colombani P, et al. Reduction of morbidity and mortality from biliary complications after liver transplantation. Hepatology. 1991;14:818–823. doi: 10.1002/hep.1840140513. [DOI] [PubMed] [Google Scholar]
- 8.Rossi G, Lucianetti A, Gridelli B, Colledan M, Caccamo L, Albani AP, et al. Biliary tract complications in 224 orthotopic liver transplantations. Transplant Proc. 1994;26:3626–3628. [PubMed] [Google Scholar]
- 9.Jeffrey GP, Brind AM, Ormonde DG, Frazer CK, Ferguson J, Bell R, et al. Management of biliary tract complications following liver transplantation. Aust N Z J Surg. 1999;69:717–722. doi: 10.1046/j.1440-1622.1999.01671.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuo PC, Lewis WD, Stokes K, Pleskow D, Simpson MA, Jenkins RL. A comparison of operation, endoscopic retrograde cholangiopancreatography, and percutaneous transhepatic cholangiography in biliary complications after hepatic transplantation. J Am Coll Surg. 1994;179:177–181. [PubMed] [Google Scholar]
- 11.Ward EM, Kiely MJ, Maus TP, Wiesner RH, Krom RA. Hilar biliary strictures after liver transplantation: cholangiography and percutaneous treatment. Radiology. 1990;177:259–263. doi: 10.1148/radiology.177.1.2399328. [DOI] [PubMed] [Google Scholar]
- 12.Sheng R, Zajko AB, Campbell WL, Abu-Elmagd K. Biliary strictures in hepatic transplants: prevalence and types in patients with primary sclerosing cholangitis vs those with other liver diseases. AJR Am J Roentgenol. 1993;161:297–300. doi: 10.2214/ajr.161.2.8333366. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Urdazpal L, Gores GJ, Ward EM, Hay E, Buckel EG, Wiesner RH, et al. Clinical outcome of ischemic-type biliary complications after liver transplantation. Transplant Proc. 1993;25:1107–1109. [PubMed] [Google Scholar]
- 14.Vorwerk D, Gunther RW, Klever P, Riesener KP, Schumpelick V. Angioplasty and stent placement for treatment of hepatic artery thrombosis following liver transplantation. J Vasc Interv Radiol. 1994;5:309–311. doi: 10.1016/s1051-0443(94)71489-5. [DOI] [PubMed] [Google Scholar]
- 15.Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40–45. doi: 10.1097/00000658-199401000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald ML, Farnell MB, Nagorney DM, Ilstrup DM, Kutch JM. Benign biliary strictures: repair and outcome with a contemporary approach. Surgery. 1995;118:582–590. doi: 10.1016/s0039-6060(05)80022-4. [DOI] [PubMed] [Google Scholar]
- 17.Zajko AB, Campbell WL, Bron KM, Lecky JW, Iwatsuki S, Shaw BW, Jr, et al. Cholangiography and interventional biliary radiology in adult liver transplantation. AJR Am J Roentgenol. 1985;144:127–133. doi: 10.2214/ajr.144.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitt HA, Kaufman SL, Coleman J, White RI, Cameron JL. Benign postoperative biliary strictures. Operate or dilate? Ann Surg. 1989;210:417–425. doi: 10.1097/00000658-198910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Culp WC, McCowan TC, Lieberman RP, Goertzen TC, LeVeen RF, Heffron TG. Biliary strictures in liver transplant recipients: treatment with metal stents. Radiology. 1996;199:339–346. doi: 10.1148/radiology.199.2.8668775. [DOI] [PubMed] [Google Scholar]
- 20.Diamond NG, Lee SP, Niblett RL, Rees CR, Klintmalm GB. Metallic stents for the treatment of intrahepatic biliary strictures after liver transplantation. J Vasc Interv Radiol. 1995;6:755–761. doi: 10.1016/s1051-0443(95)71181-2. [DOI] [PubMed] [Google Scholar]
- 21.Petersen BD, Maxfield SR, Ivancev K, Uchida BT, Rabkin JM, Rosch J. Biliary strictures in hepatic transplantation: treatment with self-expanding Z stents. J Vasc Interv Radiol. 1996;7:221–228. doi: 10.1016/s1051-0443(96)70765-0. [DOI] [PubMed] [Google Scholar]
- 22.Bonnel DH, Liguory CL, Lefebvre JF, Cornud FE. Placement of metallic stents for treatment of postoperative biliary strictures: long-term outcome in 25 patients. AJR Am J Roentgenol. 1997;169:1517–1522. doi: 10.2214/ajr.169.6.9393155. [DOI] [PubMed] [Google Scholar]
- 23.Ferrucci JT, Jr, Mueller PR, Harbin WP. Percutaneous transhepatic biliary drainage: technique, results, and applications. Radiology. 1980;135:1–13. doi: 10.1148/radiology.135.1.7360943. [DOI] [PubMed] [Google Scholar]
- 24.Letourneau JG, Hunter DW, Ascher NL, Roberts JP, Payne WD, Thompson WM, et al. Biliary complications after liver transplantation in children. Radiology. 1989;170:1095–1099. doi: 10.1148/radiology.170.3.2644669. [DOI] [PubMed] [Google Scholar]
- 25.Jan YY, Chen MF. Percutaneous trans-hepatic cholangioscopic lithotomy for hepatolithiasis: long-term results. Gastrointest Endosc. 1995;42:1–5. doi: 10.1016/s0016-5107(95)70234-2. [DOI] [PubMed] [Google Scholar]
- 26.Ponchon T, Genin G, Mitchell R, Henry L, Bory RM, Bodnar D, et al. Methods, indications, and results of percutaneous choledochoscopy. A series of 161 procedures. Ann Surg. 1996;223:26–36. doi: 10.1097/00000658-199601000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang MH, Tsai CC, Mo LR, Yang CT, Yeh YH, Yau MP, et al. Percutaneous choledochoscopic biliary tract stone removal: experience in 645 consecutive patients. Eur J Radiol. 1993;17:184–190. doi: 10.1016/0720-048x(93)90101-r. [DOI] [PubMed] [Google Scholar]
- 28.Jeng KS, Chiang HJ, Shih SC. Limitations of percutaneous transhepatic cholangioscopy in the removal of complicated biliary calculi. World J Surg. 1989;13:603–610. doi: 10.1007/BF01658880. [DOI] [PubMed] [Google Scholar]
- 29.Bonnel DH, Liguory CE, Cornud FE, Lefebvre JF. Common bile duct and intrahepatic stones: results of transhepatic electrohydraulic lithotripsy in 50 patients. Radiology. 1991;180:345–348. doi: 10.1148/radiology.180.2.2068295. [DOI] [PubMed] [Google Scholar]