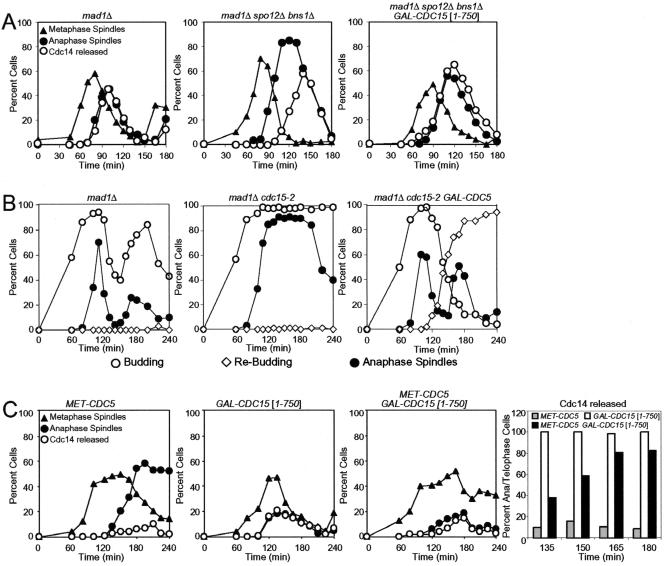
Figure 4.
Hyperactivation of the MEN can suppress the mitotic exit defect of FEAR network mutants. (A) mad1Δ (A2853), mad1Δ spo12Δ bns1Δ (A5408), and mad1Δ spo12Δ bns1Δ GAL-CDC15[1-750] (A8708) cells carrying a CDC14-3HA fusion were arrested in G1 in YEPR with α-factor (5 μg/ml) followed by released in YEPRG lacking pheromone. The percentage of cells with metaphase (closed triangles), anaphase/telophase spindles (closed circles) as well as the percentage of cells with Cdc14-HA released from the nucleolus (open circles) was determined at the indicated times. (B) mad1Δ (A2853), mad1Δ cdc15-2 (A4300), and mad1Δ cdc15-2 GAL-CDC5 (A5516) cells carrying a CDC14-3HA fusion were arrested in G1 in YEPR with α-factor (5 μg/ml) followed by released in YEPRG lacking pheromone at the restrictive temperature 37°C. The percentage of budded cells (open circles), rebudded cells (open diamonds), which indicates that cells have entered the next cell cycle as well as the percentage of anaphase/telophase spindles (closed circles) was determined at the indicated times. (C) MET-CDC5 (A9006), GAL-CDC15[1-750] (A8459), and MET-CDC5 GAL-CDC15[1-750] (A9007) cells carrying a CDC14-3HA fusion were grown in medium lacking methionine and arrested in G1 with α-factor (5 μg/ml). Methionine (8 mM) was added 1 h before release, and cells were released into YEPRG medium containing 8 mM methionine at 25°C. The percentage of cells with metaphase (closed triangles), anaphase/telophase spindles (closed circles) as well as the percentage of cells with Cdc14-HA released from the nucleolus (open circles) was determined at the indicated times. The histogram on the right shows the percentage of Cdc14 release in anaphase/telophase cells. One hundred cells were scored for each time point.