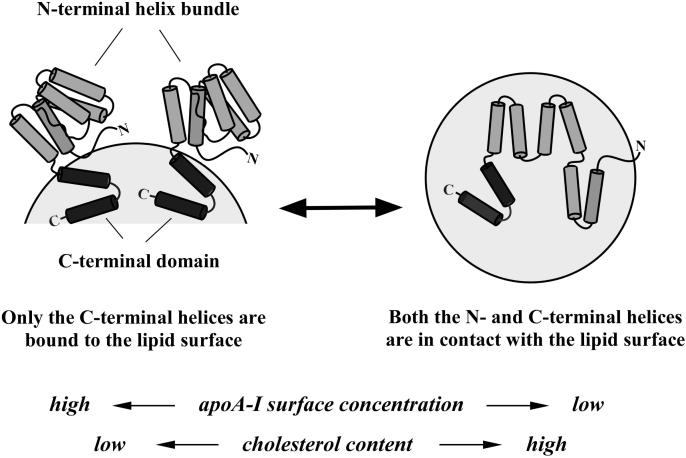
Fig. 9. Model of two possible conformations of apoA-I at the surface of a spherical lipid particle.
At high apoA-I surface concentration, the N-terminal helix bundle remains closed and out of contact with the lipid surface (left). At low surface coverage where a greater surface area is available, the helix bundle is open so that the α-helices can make contact with the lipid surface (right). Cholesterol content in the lipid surface also modulates the equilibrium between the two conformations.