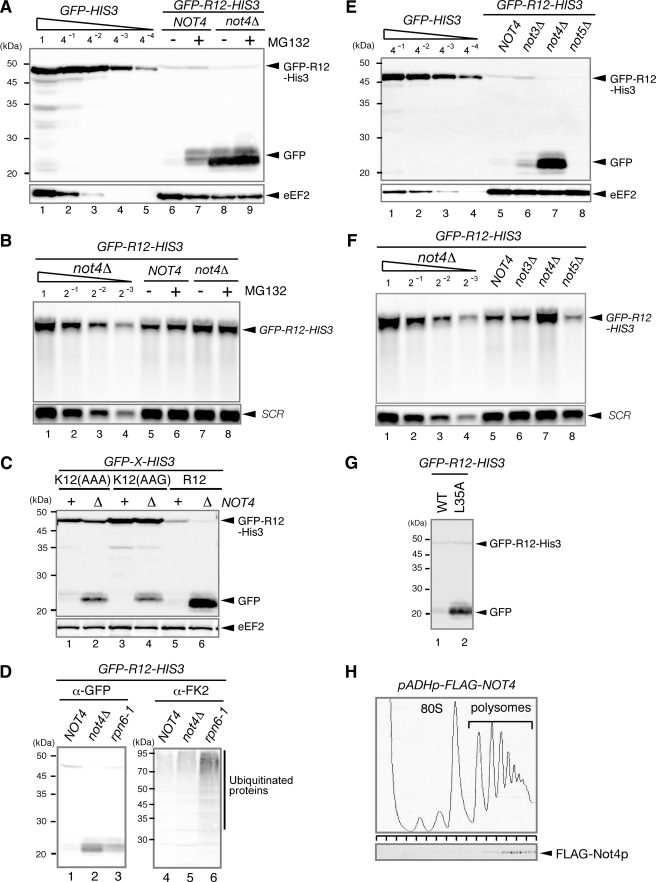
FIGURE 3.
Not4p is required for the degradation of translation arrest products by the proteasome. A, Not4p is required for the proteasome-mediated degradation of the truncated product. W303 or W303not4Δ cells were transformed with the pGPDp-GFP-R12-FLAG-HIS3 plasmid. The cells were grown in SC-Ura, and samples were analyzed using Western blotting with anti-GFP antibodies. When indicated (+), cell extracts were prepared 2 h (W303) or 5 h (W303not4Δ) after the addition of 0.2 mm MG132. B, RNA samples were prepared from cells shown in A and analyzed using Northern blotting with DIG-labeled GFP or SCR probes. C, the level of truncated product derived from GFP-K12-FLAG-HIS3 was higher in the not4Δ mutant. W303 or W303not4Δ cells were transformed with the indicated pGPDp-GFP-X-FLAG-HIS3 plasmid. The cells were grown in SC-Ura, and samples were analyzed using Western blotting. D, W303, W303not4Δ, or W303rpn6–2 (YNK7) cells harboring pGPDp-GFP-R12-FLAG-HIS3 were grown in SC medium at 30 °C. The samples were prepared and analyzed using Western blotting with anti-GFP antibodies (lanes 1–3) or anti-FK2 antibodies that recognize monoubiquitin and polyubiquitin but not free ubiquitin (lanes 4–6). E, the level of the truncated product derived from GFP-R12-FLAG-HIS3 was higher in the not4Δ mutant. W303, W303not3Δ, W303not4Δ, or W303not5Δ cells were transformed with the pGPDp-GFP-R12-FLAG-HIS3 plasmid. The cells were grown in SC-Ura, and the samples were analyzed using Western blotting. F, RNA samples were prepared from the cells shown in D and analyzed using Northern blotting with DIG-labeled GFP or SCR probes. G, the level of truncated product derived from GFP-R12-FLAG-HIS3 was higher in the not4Δ mutant expressing the Not4L35A mutant (mutation in the RING finger domain). W303not4Δ cells containing pGPDp-GFP-R12-FLAG-HIS3 were transformed with pADHp-NOT4 or pADHp-not4L35A. The cells were grown in SC-UraLeu medium, and the samples were analyzed using Western blotting. H, FLAG-Not4p protein was distributed in the polysome fractions. W303 cells were transformed with pADHp-FLAG-NOT4. Cell extracts were prepared, and polysome analysis was performed as described previously (3). Protein samples prepared from each fraction were analyzed using Western blotting with anti-FLAG antibodies. WT, wild type.