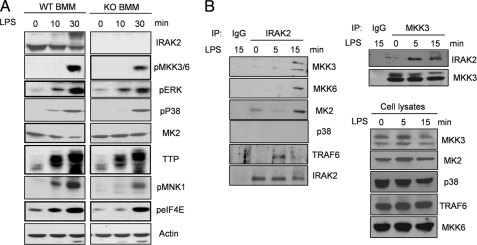
FIGURE 6.
Impaired activation of MAP kinase-mediated signaling in IRAK2-deficient macrophages in response to LPS. A, WT or IRAK2-deficient (KO) BM-derived macrophages were untreated or treated with LPS (1 μg/ml). The cell lysates were analyzed by the Western method with antibodies against IRAK2, phospho-MKK3/6, phospho-ERK, phospho-p38, MK2, TTP, phospho-MNK1, phospho-eIF4E, and actin. B, cell lysates from IRAK2 wild type or knock-out BM-derived macrophages, untreated or treated with LPS (1 μg/ml) for the indicated times, were immunoprecipitated (IP) with antibodies against IRAK2 and IgG, followed by Western analysis with antibodies against MKK3, MKK6, p38, MK2, and TRAF6. The cell lysates were also analyzed directly by the Western method with antibodies against MKK3, MKK6, p38, MK2, and TRAF6. The cell lysates from IRAK2 wild type BM-derived macrophages untreated or treated with LPS (1 μg/ml) were immunoprecipitated with antibodies against MKK3 and IgG, followed by Western analysis with antibodies against MKK3 and IRAK2.