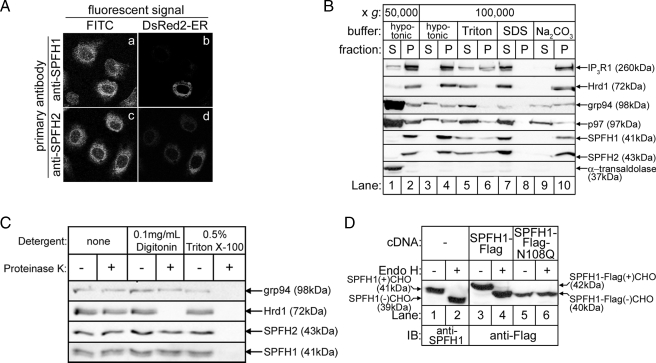
FIGURE 2.
SPFH1 and SPFH2 are type II ER membrane glycoproteins. A, Rat-1 cells plated on lysine-coated coverslips were transfected with cDNA encoding DsRed2-ER, a red fluorescent protein targeted to the ER by the calreticulin ER-targeting and KDEL ER-retention sequences. The cells were fixed and stained with anti-SPFH1 (panels a and b) or anti-SPFH2 (panels c and d), followed by fluorescein isothiocyanate (FITC)-conjugated anti-rabbit secondary antibodies as described (16). Images were acquired with a Zeiss LSM510 confocal microscope equipped with a ×63 oil immersion objective. B, essentially as described (16), αT3-1 cells were harvested in hypotonic buffer, sonicated, and fractionated into supernatant (S) and pellet (P) fractions by centrifugation at 50,000 × g for 1 h at 4 °C (lanes 1 and 2). The cytosolic protein α-transaldolase was found in the supernatant, whereas the integral membrane proteins IP3R1, Hrd1, SPFH1, and SPFH2 were found in the pellet. The peripheral membrane protein p97 and the ER luminal protein grp94 were found in both the supernatant and pellet. The 50,000 × g pellets were then resuspended in hypotonic buffer, 1% Triton X-100, 0.5% SDS, or 0.1 m Na2CO3, pH 11.2, and re-centrifuged at 100,000 × g for 1 h at 4 °C (lanes 3–10). Fractions from each centrifugation were then probed for the indicated proteins. Integral membrane proteins were released from the pellet only by detergent (lanes 5–8), whereas peripheral membrane and ER luminal proteins were released from the pellet by detergent or by Na2CO3 treatment (lanes 5–10). C, HeLa cells were treated with the indicated detergents for 10 min, followed by incubation with 1 μg/ml Proteinase K for 30 min. The reactions were quenched with 1 mm phenylmethylsulfonyl fluoride, and samples were probed for the ER luminal protein grp94, the ER membrane protein Hrd1, whose C-terminal epitope is exposed to the cytosol, SPFH1, and SPFH2. D, HeLa cells were transfected with the indicated cDNAs, and cell lysates prepared in 1% Triton X-100-containing lysis buffer were incubated without or with endoglycosidase H for 3 h to cleave N-linked glycans (CHO) and were probed with anti-SPFH1 (lanes 1 and 2) or anti-FLAG (lanes 3–6).