FIGURE 6.
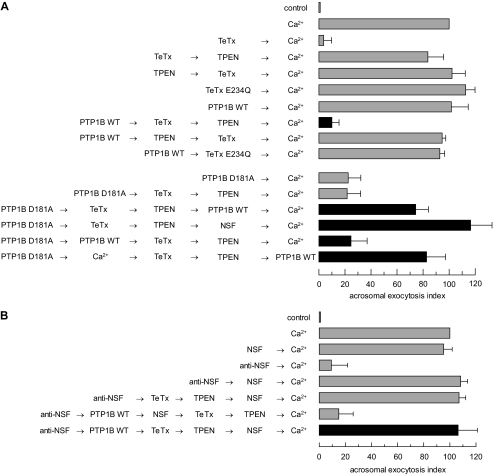
PTP1B elicits cis SNARE complex disassembly through a mechanism that requires NSF. A: top, to investigate whether PTP1B is involved in cis SNARE complex disassembly, which renders synaptobrevin sensitive to TeTx cleavage, SLO-permeabilized sperm were incubated with 27 nm wild type (WT) PTP1B for 10 min at 37 °C followed by 100 nm light chain TeTx and a further 10-min incubation at 37 °C. TeTx activity was subsequently inhibited by adding 2.5 μm TPEN. After 10 min, AR was initiated with 0.5 mm CaCl2 and incubated for 10 min at 37 °C (black bar). Bottom, SLO-permeabilized sperm were incubated with 300 nm PTP1B D181A instead of the wild type enzyme. The D181A block was released with 310 nm NSF (PTP1B D181A → TeTx → TPEN → NSF → Ca2+) or 27 nm wild type PTP1B added before (PTP1B D181A → PTP1B WT → TeTx → TPEN → Ca2+) or after (PTP1B D181A → TeTx → TPEN → PTP1B WT → Ca2+) TeTx (black bars). AR was initiated by adding 0.5 mm CaCl2. Alternatively, calcium was added after PTP1B D181A but before the toxin, TPEN, and wild type PTP1B (PTP1B D181A → Ca2+ → TeTx → TPEN → PTP1B WT, black bar). Sperm were fixed and AR was measured by FITC-PSA binding as described under “Experimental Procedures.” Controls (gray bars) included: AR inhibition by TeTx (TeTx → Ca2+) but not by its inactive mutant TeTx E234Q (43) (TeTx E234Q → Ca2+); impairing of toxin cleavage by TPEN when added before (TPEN → TeTx → Ca2+) or after (TeTx → TPEN → Ca2+) TeTx; unperturbed AR with wild type PTP1B (PTP1B WT → Ca2+); lack of monomeric synatobrevin cleavage by TeTx, despite cis SNARE complex disassembly by PTP1B WT, when added in the presence of TPEN (PTP1B WT → TPEN → TeTx → Ca2+) or when the inactive mutant replaced the wild type toxin (PTP1B WT → TeTx E234Q → Ca2+); and inhibition of the AR by PTP1B D181A in the absence (PTP1B D181A → Ca2+) or presence (PTP1B D181A → TeTx → TPEN → Ca2+) of TeTx. B, to demonstrate that PTP1B relies on NSF to disassemble cis SNARE complexes, SLO-permeabilized sperm were incubated as in A, except that an anti-NSF antibody (1:300) was added before TeTx followed by 310 nm recombinant NSF to rescue the antibody block (anti-NSF → PTP1B WT → TeTx → TPEN → NSF → Ca2+, black bar). AR was triggered as in A. Sperm were fixed and AR was measured by FITC-PSA binding as described under “Experimental Procedures.” Several controls were run (gray bars): unaffected exocytosis in the presence of NSF alone (NSF → Ca2+); inhibition of the AR by the anti-NSF antibody (anti-NSF → Ca2+) and rescue by NSF (anti-NSF → NSF → Ca2+); and lack of effect of TeTx on this rescue (anti-NSF → TeTx → TPEN→NSF→Ca2+). The combination anti-NSF/NSF did not prevent cleavage by TeTx elicited by wild type PTP1B (anti-NSF→PTP1B WT→NSF→TeTx→TPEN→Ca2+). The data were normalized as described under “Experimental Procedures.” Actual percentages of reacted sperm for control and Ca2+ ranged between 11–23 and 20–35%, respectively. The data represent the mean ± S.E. of at least three independent experiments.