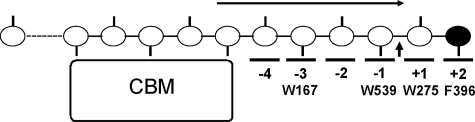
FIGURE 2.
Schematic picture of ChiA from S. marcescens in complex with a single chitin chain. The catalytic domain of the enzyme has six subsites, numbered from -4 to +2. CBM indicates a FnIII-type carbohydrate-binding module thought to be involved in chitin-binding (12). The reducing end sugar is colored black. Approximate positions of important aromatic residues that have hydrophobic interactions with sugar residues are shown. The glycosidic bond between the sugar residues in subsite +1 and -1 is enzymatically cleaved (illustrated with an arrow). A correctly positioned N-acetyl group (shown as sticks) in the -1 subsite is essential for the substrate-assisted catalysis (35–37). The scheme shows the situation during processive action when only dimers are produced. The arrow indicates the supposed direction of the sliding of the substrate through the active site cleft (48, 49). In the case of chitosan, which consists of both N-acetylated glucosamines (GlcNAc or A units) and glucosamines (GlcN or D units), complexes formed during processive action may be nonproductive because the sugar bound in the -1 subsite may lack the catalytically crucial N-acetyl group. This leads to the production of longer even-numbered oligomers, which is diagnostic for processivity (30, 31, 38).