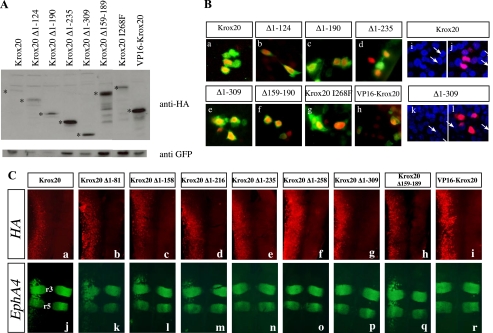
FIGURE 3.
Deleted Krox20 proteins are efficiently expressed and localized in the nucleus. A, estimation of the relative amounts of Krox20 mutant proteins following electroporation. Chick embryo neural tubes were co-electroporated with each of the HA-tagged Krox20 deletion mutant plasmids and a GFP expression vector (pEGFP-N1) (43). Western blotting was then performed using an anti-HA antibody and an anti-GFP antibody for normalization. The bona fide deleted Krox20 proteins are marked with asterisks. Their relative amounts are higher than or equivalent to that of the wild type protein. B, subcellular localization of the deleted Krox20 proteins. Chick embryo neural tubes were co-electroporated with pEGFP-N1 and constructs encoding wild type or mutant HA-tagged Krox20, as indicated. Double immunolabeling against HA and GFP was performed and the nuclei were counterstained with Hoechst 33342. Comparisons of anti-HA (red) and anti-GFP (green) staining (a-h) or of anti-HA (red) and Hoechst (blue) staining (i-l) indicate that the various Krox20 mutants are essentially nuclear. i and k, Hoechst staining only. a, i, and j correspond to the same field as e, k, and l. The arrows point to the nuclei positive for Krox20 in these latter cases. C, ectopic target gene expression reflects mutant Krox20 protein transcriptional activity. Chick embryo neural tubes were electroporated on the left side with wild type or truncated HA-tagged Krox20 expression constructs, as indicated, double-immunolabeled for the HA epitope (a-i; red) and the EphA4 protein (j-r; green), and flat mounted. These data establish that loss of transactivation by constructs having lost the 235 N-terminal amino acids is not due to lack of expression. It also shows that the anterior-posterior restriction of the EphA4 ectopic expression domain is not accounted for by the area of efficient electroporation.