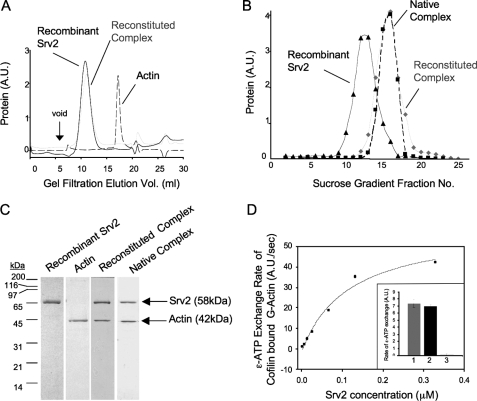
FIGURE 1.
Reconstitution of the Srv2-actin complex from purified proteins. A, gel filtration analysis of purified full-length recombinant Srv2, actin, and reconstituted Srv2-actin complex. B, sucrose gradient sedimentation analysis of recombinant full-length Srv2, reconstituted Srv2-actin complex, and native Srv2-actin complex. C, Coomassie-stained gel of peak fractions from gel filtration compared with native Srv2-actin complex isolated from yeast. D, recombinant Srv2 has concentration-dependent effects in relieving cofilin inhibition of ε-ATP nucleotide exchange on actin monomers. Inset, ε-ATP exchange rates for reactions containing 1 μm G-actin, 2 μm Cof1, and either 53 nm recombinant Srv2 (bar 1), 53 nm native Srv2 (bar 2), or no Srv2 (bar 3). A.U., arbitrary units.